Colon-targeted delivery systems of budesonide as second-line therapy in inflammatory bowel disease
Abstract
In inflammatory bowel disease (IBD), some parts of the gastrointestinal tract GIT become inflamed, depending on the location of the inflammation, it’s divided into two types Crohn’s and ulcerative colitis (UC). The local absorption of anti-inflammatory drugs such as budesonide (BUD) at the site of involvement leads to a reduction in the symptoms of UC. However, GIT is very complex and many physiological obstacles can be challenging in delivering drugs to the colon. BUD is a topical glucocorticosteroid used as a second-line treatment for UC. Common commercial brands of BUD, including Budenofalk®, Entocort® and Cortiment® are often ineffective in targeting the colon due to their high dependence on GIT physiological variables. To deliver BUD to the colon, the drug formulation should be formulated so that it prevents the release of the drug in the upper GIT and starts releasing the drug content as soon as it reaches the colon. Various approaches, including the modifying of pharmaceutical formulations using drug delivery systems dependent on microbial degradation, time-dependent and pH-dependent, have been investigated separately or in combination with each other. In the present study, different strategies used in the colon delivery of BUD have been reviewed.
Introduction
IBD is a chronic digestive disease caused by intestinal disorders that cause long-term inflammation of the GIT [1,2]. IBD is divided into two types: ulcerative colitis (UC) and Crohn’s [3]. UC occurs when the lining of the colon, rectum, or both become inflamed, resulting in superficial ulcers in these areas, while in Crohn’s disease, various parts of the GIT from the mouth to the anus can be involved in inflammation [4]. Crohn’s disease mostly affects the end part of the small intestine and the beginning part of the large intestine and can involve all layers of the intestine and even cause fibrosis of the intestinal tissue, although there are usually healthy areas between the lesions [4].
To treat IBD, various systems have been designed to deliver drugs to the colon and small intestine [2,5]. The main mechanism of these systems is based on changes in the physiological factors of the GIT, such as pH, residence time of the dosage form, and microbial flora in the GIT [3,6]. In newer systems, it has been tried to combine different mechanisms [7]. The advantage of these systems is that the dependence of the combined system on changes in the physiological conditions of the GIT will be less, and as a result, drug release from the system will be more predictable in different conditions [8].
Mesalazine or 5-ASA, BUD, thiopurines, sulfasalazine, and methotrexate are common treatments for IBD [[9], [10], [11]]. However, mesalazine and BUD are used as the first and second lines of therapy, respectively [3,12]. In recent years, scientific and clinical attention has focused on BUD, which is extensively metabolized systemically in the liver and intestinal wall [[13], [14], [15]]. BUD is a potent corticosteroid, and its commercial formulations include Budenofalk® and Entocort® EC, which deliver the drug to the ileum and the beginning of the colon [16]. However, continuous use of BUD does not lead to long-term recovery, and it is better to use it intermittently to treat acute exacerbations [14,17]. It has been reported that BUD inhibits neutrophil apoptosis, NF-Kappa B, phospholipase A2, and inflammatory transcription factors [18]. This can suppress the immune response and reduce symptoms associated with certain inflammatory conditions [18]. However, inhibition of neutrophil apoptosis may impair the clearance of pathogens and increase the risk of infection [18]. It should be noted that low doses of BUD produce an anti-inflammatory effect, and the use of higher doses leads to the suppression of the immune system.
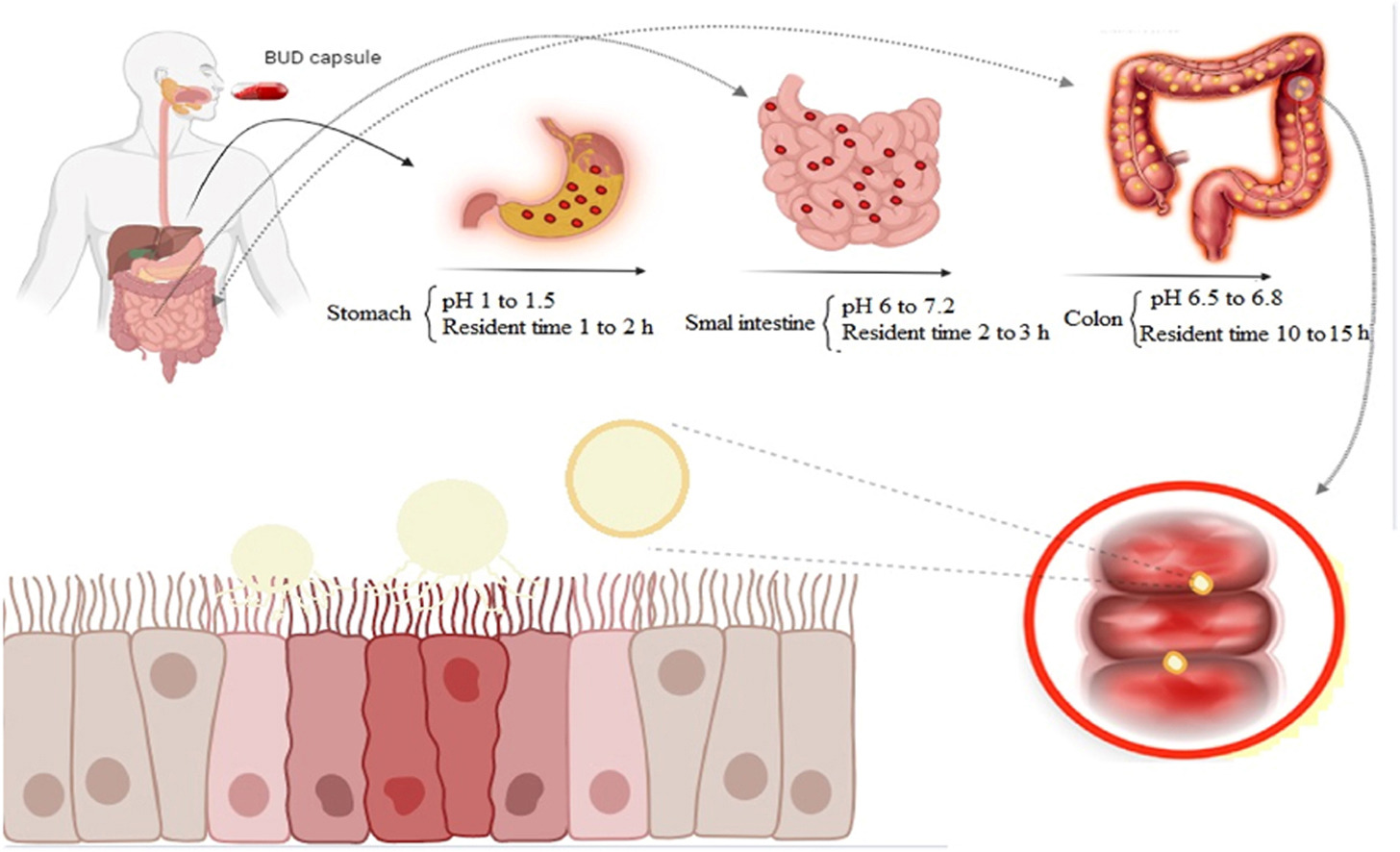
Because the long-term use of BUD with high doses causes the drug to bind to the mineralocorticoid receptor and will eventually lead to an increase in sodium levels and a decrease in potassium levels [19]. If BUD is taken orally, the drug is absorbed in the upper GIT and leads to side effects such as heart palpitations, muscle cramps, headache, fatigue, abdominal pain and itching or redness of the skin [20]. On the other hand, drug absorption in the upper parts of the GIT causes a minimal amount of the drug to reach the end parts of the GIT, which leads to a low local concentration of the drug in the inflammation area. Consequently, the effectiveness of the treatment decreases [4]. Therefore, depending on the type of IBD, targeting slow-release BUD to inflamed areas can reduce these problems [[21], [22], [23]]. In the design of slow-release formulations, should be a complete comprehension of the anatomy and physiology of the GIT [2,11]. However, pH and residence time in each part of GIT, as well as microbial flora are the most critical factors that should be considered in designing the dosage forms [24]. The pH of different parts of the GIT, including the stomach, duodenum, jejunum, terminal ileum, and colon is reported to be about 1.2, 6.5, 6.8, 7.2 and 6.8, respectively [25]. Reports show that it takes about 2, 3, and 10 h for the dosage form to pass through the stomach, small intestine, and large intestine, respectively [23,25].
In other words, the residence time of the dosage form in the GIT of a patient with UC remains about 15 h [25]. Therefore, BUD colon delivery formulation should be designed in such a way that it starts releasing drug content in the colon region with a delay phase of about 5 h and releases the entire drug content in this region within 8–10 h at most. The gradual release of BUD in the colon area allows the throughout the colon to be accessed the drug [[25], [26], [27]]. Some studies have also been done in targeted BUD delivery based on polysaccharides [[28], [29], [30]]. Polysaccharides have been widely investigated in colon drug delivery due to their natural abundance, cheap price, safety, non-toxicity, biodegradability and sensitivity to bacterial enzymes [31,32]. Polysaccharides such as pectin, chitosan, guar, dextran and inulin disintegrate in the colon due to the presence of glucosidase, nitroreductase, glucuronidase, azoreductase, esterases and amidase enzymes produced by Bifidobacteria, Bacteroides, Escherichia coli, Clostridium, Enterobacteriaceae and Lactobacillus [[33], [34], [35], [36], [37]].
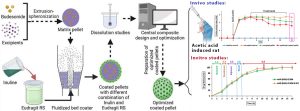
Therefore, polysaccharides have the potential to use in the matrix or coat of BUD dosage forms [33]. New approaches in the colonic delivery of BUD have tended towards multi-unit systems (pellets) instead of single-unit systems such as tablets [[38], [39], [40]]. Pellets are spherical objects with a diameter between 0.1 and 2 mm, which have attracted attention for colonic delivery of drugs due to their uniform particle size distribution, excellent flowability, smooth surface for coating, the fast passage through the upper parts of the GIT [40]. In addition, pellets reduce the risk of dumping doses, which can reduce the side effects of drugs and improve bioavailability [41]. To overcome the physiological conditions of the GIT, in recent years, the attention of researchers has been drawn towards systems based on micro and nanoparticles [42,43]. However, targeted drug delivery of BUD to the colon with these systems requires the use of polymers dependent on pH, time and microbial degradation separately or in combination with each other in the structure of these dosage forms [3,18,42,44]. In this regard, in the current review, all studies based on efficient BUD colon delivery systems to treat IBD are discussed.
Read more here
Hossein Shahdadi Sardou, Mohammadreza Abbaspour, Abbas Akhgari, Prashant Kesharwani, Amirhossein Sahebkar,
Colon-targeted delivery systems of budesonide as second-line therapy in inflammatory bowel disease, Journal of Drug Delivery Science and Technology, Volume 93, 2024, 105472, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105472.
Read more interesting articles about the use of Budesonide here:
- Comparative Dissolution of Budesonide from Four Commercially Available Products for Oral Administration
- Preparation and evaluation of β-cyclodextrin-based nanosponges loaded with Budesonide for pulmonary delivery
- Combination of time-dependent polymer and inulin as a coating for sustained delivery of budesonide pellets aimed for use in IBD treatment