Drug delivery to the brain: In situ gelling formulation enhances carbamazepine diffusion through nasal mucosa models with mucin
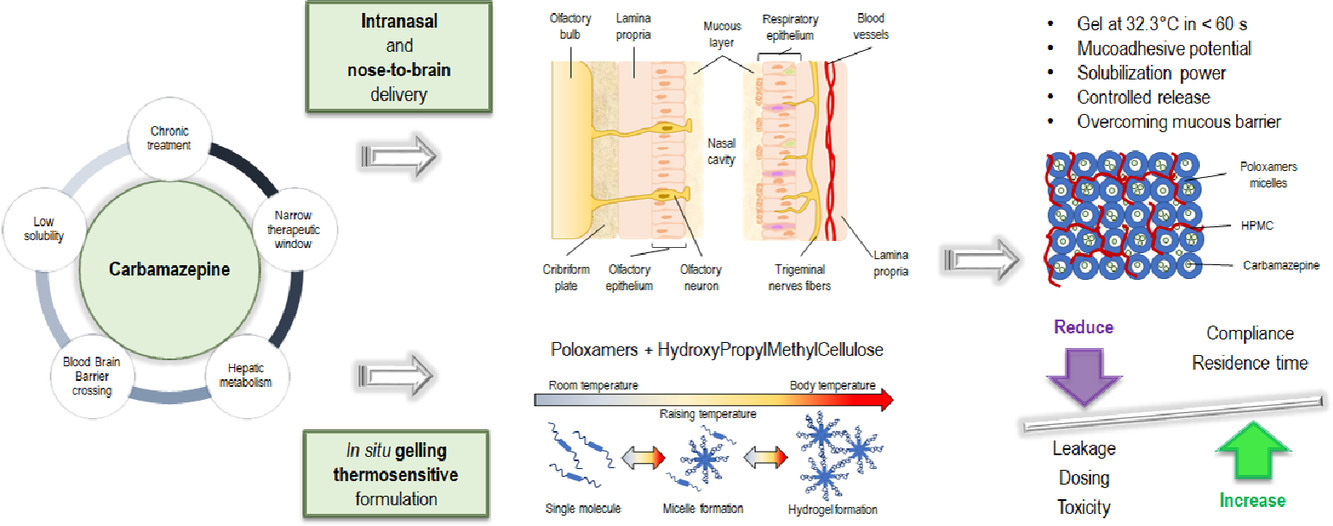
The objective of this work was to optimize a thermosensitive in situ gelling formulation to improve intranasal and nose-to-brain delivery of the antiepileptic drug carbamazepine (CBZ). A preliminary procedure of vehicles obtained just mixing different fractions of poloxamer 407 (P407) and poloxamer 188 (P188) revealed preparations with phase transition temperatures, times to gelation and pH values suitable for nasal delivery. Subsequently, the mucoadhesive properties of the most promising formulations were tuned by adding hydroxypropylmethylcellulose types of different viscosity grades, and the effect of the adhesive polymers was evaluated by testing in vitro time and strength of mucoadhesion on specimens of sheep nasal mucosa.
The formulation that showed the greatest mucoadhesive potential in vitro, with a time and force of mucoadhesion equal to 1746,75 s and 3.66 × 10-4 N, respectively, was that composed of 22% P407, 5% P188 and 0.8% HPMC low-viscous and it was further investigated for its ability to increase drug solubility and to control the release of the drug. Lastly, the capability of the candidate vehicle to ensure drug permeation across the biomimetic membrane Permeapad®, an artificial phospholipid-based barrier with a stratified architecture, and the same barrier enriched with a mucin layer was verified.
The final formulation was characterized by a pH value of 6.0, underwent gelation at 32.33°C in 37.85 s, thus showing all the features required by in situ gelling thermosensitive preparations designed for nasal delivery and, more notably, it conserved the ability to favor drug permeation in the presence of mucin. These findings suggest that the optimized gelling system could be a promising and easy to realize strategy to improve CBZ delivery to the brain exploiting both a direct and indirect pathway.
Download the full article as PDF here Drug delivery to the brain_In situ gelling formulation enhances carbamazepine diffusion through nasal mucosa models with mucin
or read it here
Materials
CBZ was provided from Sigma-Aldrich (Søborg, Denmark), whereas Lutrol® F68 (P188) and Lutrol® F127 (P407) were a kind gift from BASF SE (Ludwigshafen, Germany). HPMC high-viscous Benecel™ K100M Pharm (75000-140000 cps at 2% w/w) (K100M) and low-viscous Methocel™ E50LV Premium 5P (35-65 cps at 2% w/w) (E50LV) were supplied by Ashland Industries Europe GmbH (Schaffhausen, Switzerland) and Sigma-Aldrich (Milan, Italy), respectively. Green food coloring E-102 E-131 was provided from Candi Gestro srl (Siderno, Italy). Mucin type II from porcine stomach, all chemicals, and solvents were of analytical grade and purchased from Sigma-Aldrich (Milan, Italy), except for sodium chloride (NaCl) that was supplied by Carlo Erba (Milan, Italy). Phosphate buffer solution (PBS) at pH 7.4 was composed of 7.4 mM Na2HPO4·12H2O, 1.1 mM KH2PO4, and 136 mM NaCl. PBS at pH 5.5 was employed to simulate the pH of the nasal cavity and it was composed of 4.2 mM Na2HPO4·12H2O, 100 mM KH2PO4, 45.5 mM NaCl.
Elisa Corazza, Massimiliano Pio di Cagno, Annette Bauer-Brandl, Angela Abruzzo, Teresa Cerchiara, Federica Bigucci, Barbara Luppi, Drug delivery to the brain: In situ gelling formulation enhances carbamazepine diffusion through nasal mucosa models with mucin, European Journal of Pharmaceutical Sciences, Volume 179, 2022, 106294, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2022.106294

