Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery
Abstract
The aim of the study was the preparation and comparison of two types of orodispersible gel films (ODF) by the solvent casting method. Natural polymers: sodium alginate (ALG) or hydroxypropyl methylcellulose (HPMC) were used as the gel film formers, and Kollidon or microcrystalline cellulose was used as the disintegrant. Meloxicam (MLX), the drug used to treat rheumatic diseases for children and adults, was proposed as the active pharmaceutical ingredient (API). The influence of the polymer and disintegrant on the properties of ODF was investigated. The evaluation of prepared gel films was based on appearance description, mass uniformity measurement, disintegration time, API content, film wettability, and water content. Also, the dissolution test was prepared in a basket apparatus using artificial salvia (pH = 6.8) as the medium. The obtained API release profiles were analyzed for the similarity factors (f2) with the DDSolver software. The results showed that independently of the polymer or disintegrant, using the solvent casting method, gel films have a similar appearance and active substance content close to the theoretical value and water content of less than 10%. Only the type of polymer influences the release profiles of MLX. However, the disintegration time was longer than 30 s, which makes the films non-fast-dissolving drug delivery systems. This means that for the ODF system, further evaluation is required, and some changes in the composition of the film have to be done.
Introduction
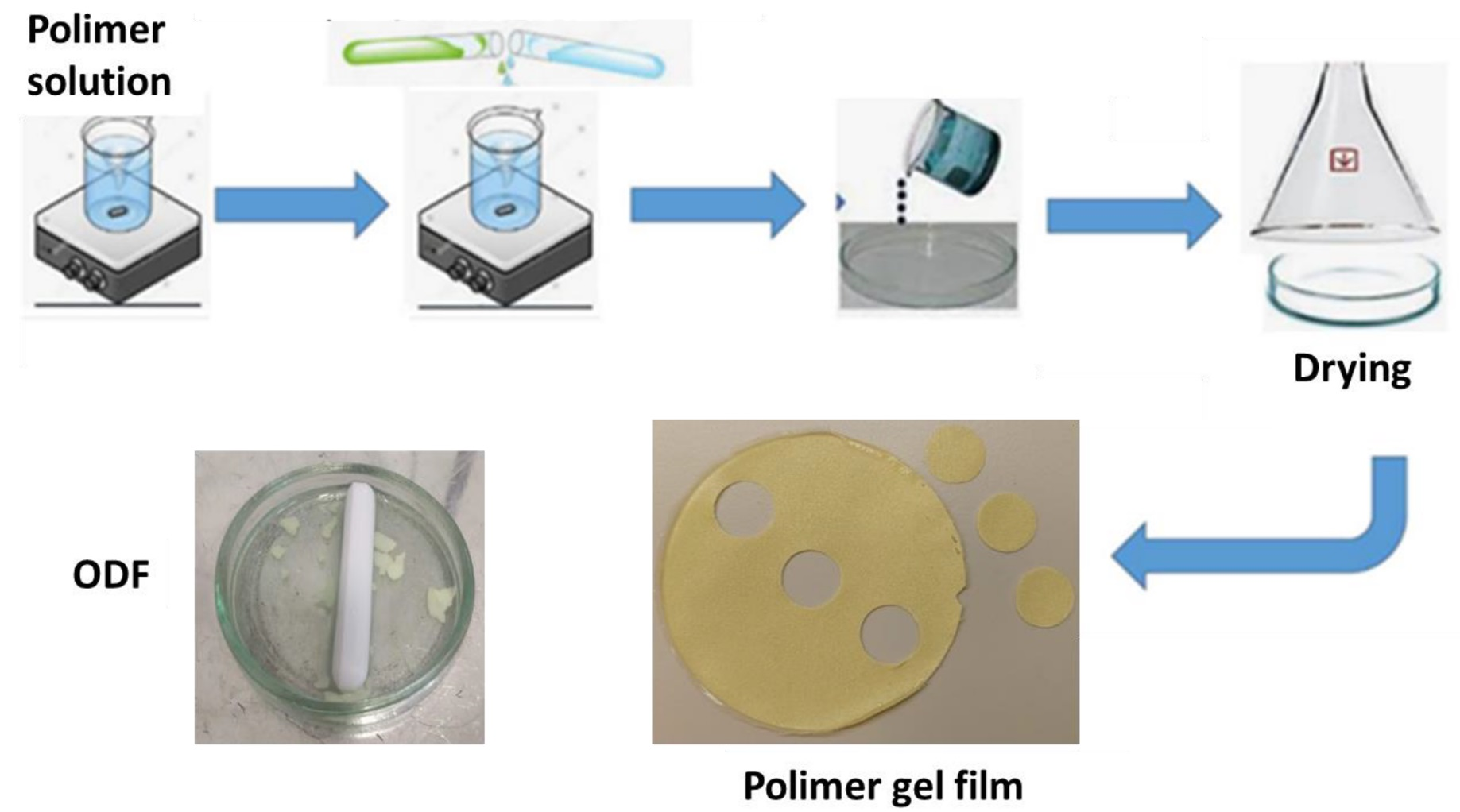
Polymer gel films, also known as polymer films, orally disintegrating membranes or films, lamellae, or ODF (Oro-dispersible films) are oral drug forms. They can be found as oblong, round or other shapes with an area of 2 to 8 cm2, are usually flat with a thickness ranging from 12 to 100 µm, and consist of one or more polymer gel layers [1,2,3,4,5,6,7]. As many as 37% of surveyed patients have difficulty swallowing tablets and capsules. The advantage of using drugs in the form of orodispersible polymer films is that there is no need to swallow them, which works well for children, the elderly, or patients with dysphagia [4,5,6]. They are placed in the oral cavity, where they should disintegrate in approximately 30 s and release the active substance [1]. After the oral membrane breaks down, the released API is swallowed by the patient, and then the substance is absorbed into the bloodstream. Taking the drug in this form does not require chewing or drinking water; the gel film is placed on the tongue, and its disintegration occurs after contact with saliva in the oral cavity [3,4,5,7]. According to the FDA (Food and Drug Agency) [8], 4% of patients cease taking medications in the form of tablets due to problems with swallowing. ODFs are a form perfectly suitable for elderly patients, children, people who have problems or fears related to swallowing, patients after surgery of the upper sections of the gastrointestinal tract, patients who do not cooperate, or patients in places without access to drinking water. Compared to orally-disintegrating tablets, polymer films are flexible and do not crumble, facilitating their dosing and taking up less space [9,10,11]. When using film strips, there is no risk of taking the wrong dose, as in the case of dispensing liquid forms of drugs, because they contain a precisely measured amount of the drug in one portion. There is no risk of choking when using ODFs. The limitation that can be faced when choosing a drug in the form of polymer gel membranes is their capacity. Due to their size, the lamellas can contain small doses of medicinal substances, which is why they are good for strong APIs [12,13,14,15]. Due to the release of the drug in the mouth, as well as the fact that the taste of API is often not pleasant, there is a need to use flavor enhancers, which additionally reduce the capacity of films for medicinal substances [3,6]. Another limitation is the lack of stability of the gel films in a humid environment. ODFs are also not used for substances that are unstable in the pH of the oral cavity or substances that irritate the area of the oral cavity and tongue [5]. The lamellas consist primarily of API, a properly selected polymer, disintegrants, plasticizers, and substances that improve the appearance and taste [6]. ODFs are an attractive and innovative drug form that differ from traditional and popular tablets and other known formulations [6,11,14]. They usually contain strong substances that have a sweet taste thanks to the auxiliary ingredients and can look attractive thanks to the use of dyes. They can be used at any time and under any conditions. Many methods of producing lamellas have already been determined, and one of the most commonly used is the solvent-casting method. It owes its popularity to the simplicity of execution and the lack of the need to use special technologies for production. The resulting solution or suspension, with the appropriate composition, is poured into Petri dishes or appropriate molds and then left to dry. Larger films are created, from which smaller ones of precisely defined dimensions are cut out and packed into protective films [7].
The composition of polymer gel films, apart from the drug and the polymer, includes plasticizers, taste masking agents, dyes, and surfactants [13,15]. The most important role in the production of films is played by polymers, which ensure the flexibility of the resulting gel films and allow them to disintegrate when placed in the oral cavity. The polymers selected for the production of films must be thoroughly tested. The most frequently chosen polymers are HPMC (Hydroxypropyl Methyl Cellulose) and HPC (Hydroxypropyl Cellulose). They show good film-forming properties and are widely used in the pharmaceutical industry as polymers for coating tablets, granules, or pellets [8,9,11,12,13,14,15].
The aim of the presented research was to prepare polymer gel films/membranes using the solvent-casting technique, which could be used for orodispersible drug delivery. The following polymers were used: sodium alginate or hydroxypropyl methylcellulose, and the effect of microcrystalline cellulose (mcc) and kollidon (koll), i.e., so-called disintegrators, on the rate of disintegration of the tested films were also investigated [16,17,18,19].
HPMC, otherwise known as hypromellose, belongs to cellulose derivatives (Figure 1). Cellulose is one of the common, natural polymers, which is a linear polysaccharide built of D-glucopyranose units linked by 1,4-β glycosidic bonds. HPMC is a cellulose ether, which is created by replacing the hydroxyl groups of cellulose with methyl and hydroxypropyl groups [16,17].
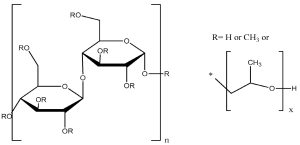
HPMC dissolves in cold water; when heated to 50–80 °C, it forms a thermally reversible, hard gel. It is insoluble in ethanol and other organic solvents. A pure HPMC solution without additives has a pH of approximately 6.47–7.87 [17].
Sodium alginate is a linear copolymer of β-D-mannuronic acid (M) residues linked with α-L-guluronic acid (G) with 1,4-glycosidic bonds (Figure 2).
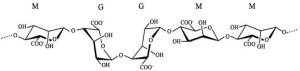
The number of M and G blocks, as well as their distribution, affects the physicochemical properties of alginate. An abundance of M blocks provides a flexible structure and biocompatibility, while a greater amount of G blocks shows a stiffer structure. Alginate has hydroxyl and carboxyl groups located along the skeleton, which can crosslink to form hydrogels [18,19]. It forms a viscous, colloidal solution in water. It is insoluble in chloroform, ether, and in aqueous solution below pH = 3. This compound has excellent filming properties [18].
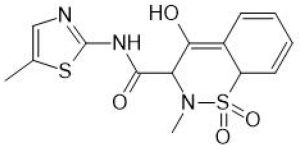
The drug substance selected for the study was meloxicam (Figure 3), a cyclooxygenase inhibitor (COX-2-induced) with analgesic, anti-inflammatory, and antipyretic properties, mainly used in the course of rheumatic diseases in adults and children [20,21,22,23]. It is a drug from the group of non-steroidal anti-inflammatory drugs (NSAIDs) belonging to oxicams, classified as class II BCS (Biopharmaceutical Classification System), which means that it is characterized by low solubility and good permeability, the main characteristics are shown in Table 1.
The evaluation of prepared gel films was based on appearance description, mass uniformity measurement, disintegration time, API content, film wettability, and water content. The dissolution of MLX from prepared gel films was carried out in a basket apparatus using artificial salvia (pH = 6.8). Additionally, API release profiles were analyzed for similarity factors (f2) with DDSolver software.
Download the full article as PDF here Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery
or read more here
Materials
The model drug Meloxicam (MLX) was a gift from Biofarm (Poznan, Poland) and was used as received. Sodium alginate was purchased from Sigma-Aldrich, HPMC was purchased from Colorcon Limited, Kollidon CL from BASF ChemTrade GmbH, and MCC-Emocel 90XLM was purchased from JRS Pharma. Sodium hydroxide, sodium chloride, and kalium dihydrophosphate were purchased from Avantor Performance Materials S.A. Disodium bicarbonate and phosphate acid were received from Chempur.
Jadach, B.; Misek, M.; Ferlak, J. Comparison of Hydroxypropyl Methylcellulose and Alginate Gel Films with Meloxicam as Fast Orodispersible Drug Delivery. Gels 2023, 9, 687. https://doi.org/10.3390/gels9090687

