Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications

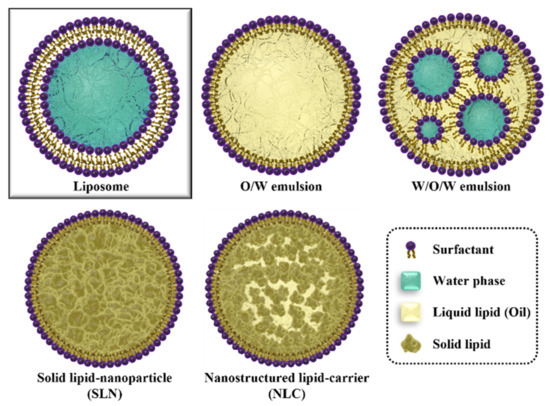
Numerous drugs have emerged to treat various diseases, such as COVID-19, cancer, and protect human health. Approximately 40% of them are lipophilic and are used for treating diseases through various delivery routes, including skin absorption, oral administration, and injection. However, as lipophilic drugs have a low solubility in the human body, drug delivery systems (DDSs) are being actively developed to increase drug bioavailability. Liposomes, micro-sponges, and polymer-based nanoparticles have been proposed as DDS carriers for lipophilic drugs.
However, their instability, cytotoxicity, and lack of targeting ability limit their commercialization. Lipid nanoparticles (LNPs) have fewer side effects, excellent biocompatibility, and high physical stability. LNPs are considered efficient vehicles of lipophilic drugs owing to their lipid-based internal structure. In addition, recent LNP studies suggest that the bioavailability of LNP can be increased through surface modifications, such as PEGylation, chitosan, and surfactant protein coating. Thus, their combinations have an abundant utilization potential in the fields of DDSs for carrying lipophilic drugs. In this review, the functions and efficiencies of various types of LNPs and surface modifications developed to optimize lipophilic drug delivery are discussed.
1. Introduction
Download the full study as PDF here: Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications
or read it here
Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772.
https://doi.org/10.3390/pharmaceutics15030772
Read more on Chitosan as a pharmaceutical excipient here:


