Microneedles assisted controlled and improved transdermal delivery of high molecular drugs via in situ forming depot thermoresponsive poloxamers gels in skin microchannels
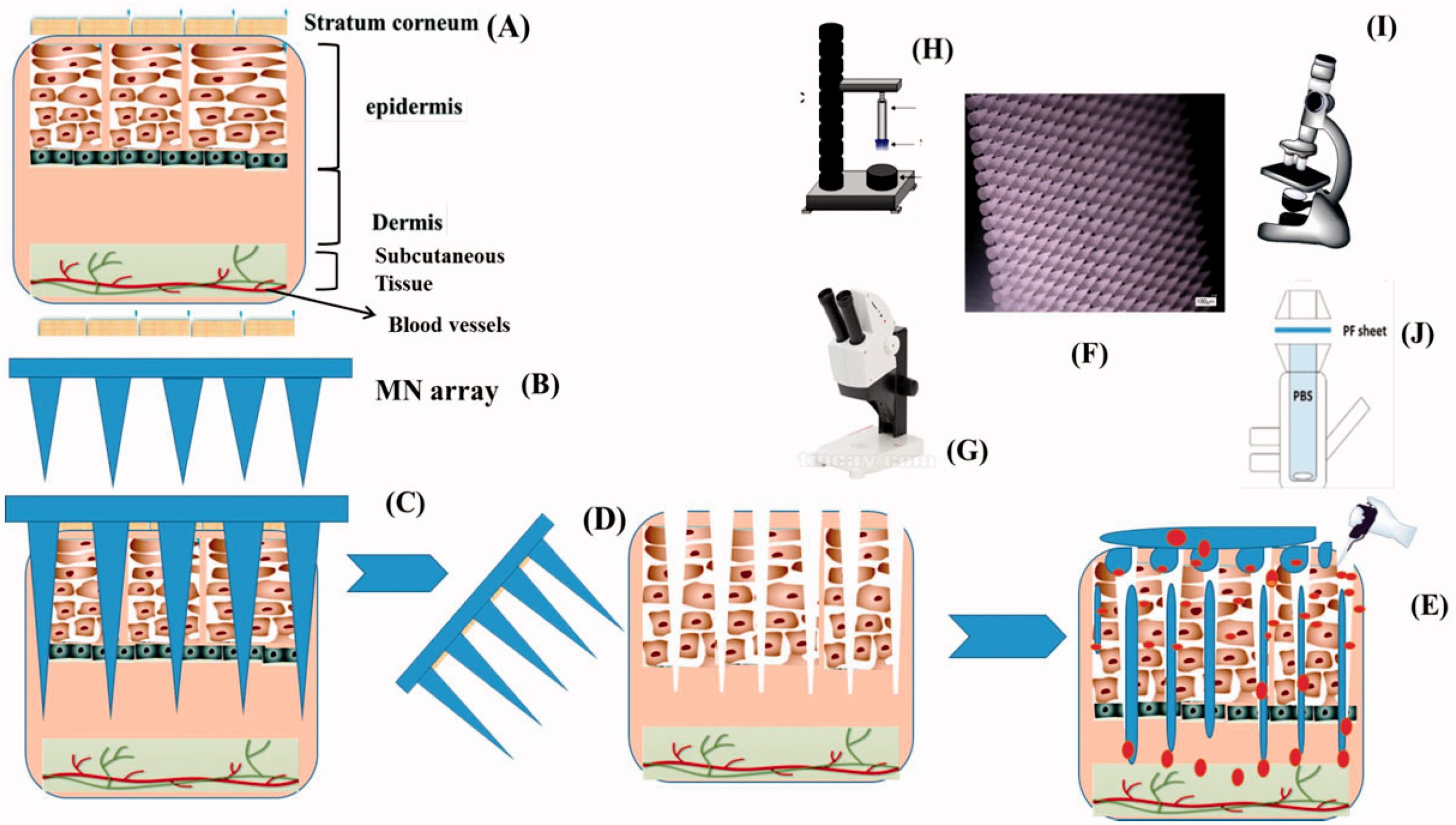
Skin is considered as an attractive route for variety of drug molecule administration. However, it is proved to be the main physical barrier for drug flux owing to their poor permeability and low bioavailability across stratum corneum layer. In the current study, novel approach has been used to enhance transdermal delivery via microporation through combination of poloxamers gels and microneedles (MNs) arrays. The phase transition of poloxamers at various concentrations from sol–gel was evaluated using AR2000 rheometer to confirm MNs-assisted in situ forming depots. Temperature test confirmed gelation between 32 and 37 °C. Curcumin was loaded in poloxamer formulations at variable concentrations and its effect showed reduction in critical gelation temperature (CGT) owing to its hydrophobic nature.
Microneedle arrays (600 µm) prepared from Gantrez S-97, PEG10000 and gelatin B using (19 × 19) laser-engineered silicone micromoulds showed high mechanical stability investigated via Texture analyzer. From in situ dissolution profile, gelatin 15% w/w based MNs displayed quicker dissolution rate in comparison to PG10000. VivoSight® OCT scanner and dye tracking confirmed that PG10000 MNs arrays pierced SC layer, infiltrate the epidermis and goes to dermis layer. From in vitro permeation, it was concluded that 20% w/w PF127® gel formulations containing (0.1% and 0.3%) curcumin displayed high curcumin permeation for comparatively longer time through microporated skin samples in comparison to non-microporated skin. The curcumin distribution in skin tissues with higher florescence intensity was noted in MNs treated skin samples by confocal microscopy. FTIR confirmed the structure formation of fabricated MNs, while TGA showed dry, brittle and rigid nature of gelatin MNs.
In this study, we report the formation of MNs assisted in situ forming hydrogel depots in the skin micro-channels using a range of poloxamers (poloxamer 407 or Pluronic® PF127 and poloxamer P87) and utilizing their temperature responsive sol–gel transition properties. Current study was based on the hypothesis that skin will be microporated by using optimized MN arrays followed by MNs assembly removal and application of drug loaded poloxamer solutions. The poloxamer drug containing solution will flow inside the pores and convert into depot at skin temperature (32 °C) gaining the MN shape and will provide controlled delivery of high molecular weight loaded molecules. In the current study, first different poloxamer grade solutions at variable concentrations were prepared and subjected to rheological analysis for the confirmation of sol–gel transition temperatures (32–37 °C). Then, 19 × 19 MN arrays (600 µm, height) were fabricated from aqueous solution of 15% w/w Gantrez S97/7.5% w/w, poly(ethyleneglycol) (PEG) 10,000 Da and 15% gelatin B. The MNs were screened for mechanical strength, in situ dissolution profile and moisture contents determination.
The histology of piglet skin for pores creation by MN arrays application was investigated by dye tracking study and optical coherence tomography (OCT). The MNs insertion depth in hairless skin was also observed by OCT. The porcine skin barrier integrity was observed with trans-epidermal water loss (TEWL) before and after MNs treatment. Curcumin as a model pharmaceutical was loaded in optimized poloxamer solutions. To the best of our knowledge and as per literature review, to date, no study has been conducted for curcumin delivery through transdermal route using MNs assisted in situ forming hydrogel depots. The in vitro permeation of curcumin across skin samples was observed using vertical Franz’s cell diffusion. Furthermore, confocal microscopy was used to track the biodistribution of curcumin in the skin layers. The MNs structural assessment was studied by Fourier transform infrared spectroscopy (FT-IR), while morphology of the MNs was assessed by scanning electron microscopy (SEM). Figure 1 refers to schematic diagram of in situ gel design and drug delivery from MNs assisted in situ forming gel depot.
Download the full article as PDF here Microneedles assisted controlled and improved transdermal delivery of high molecular drugs via in situ forming depot thermoresponsive poloxamers gels in skin microchannels
or read it here
(2022) Microneedles assisted controlled and improved transdermal delivery of high molecular drugs via in situ forming depot thermoresponsive poloxamers gels in skin microchannels, Drug Development and Industrial Pharmacy, https://doi.org/10.1080/03639045.2022.2107662

