Existing and emerging mitigation strategies for the prevention of accidental overdose from oral pharmaceutical products Review
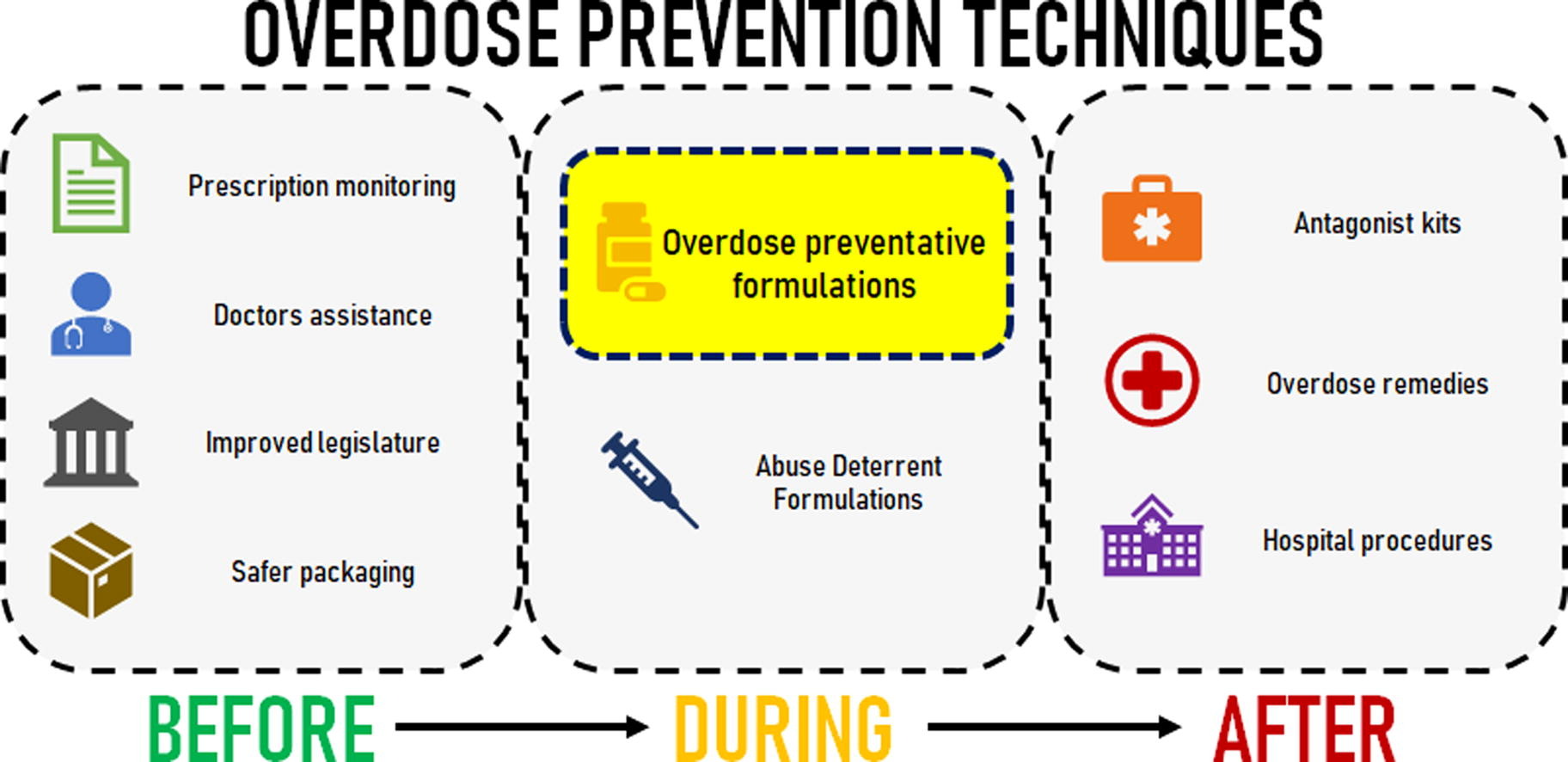
Misadventure with pharmaceutical oral medication has been on the rise, with the opioid crisis playing a major part. Drug overdose related to opioids has become such an issue, that it has been labelled a worldwide crisis. This review explores the mitigation strategies currently in place to prevent accidental overdose from oral pharmaceuticals, categorising the options based on whether they are relevant before, during or after the consumption of a toxic drug dose. To prophylactically prevent an overdose before consumption, governments and medical boards provide guidelines and implement policy, such as prescription monitoring, for the use of heavily abused medication. Some opioids have also been formulated as abuse deterrent formulations (ADF) which make it difficult for an individual to tamper with the medication. However, this does not prevent accidental overdose and only a few novel formulations were found to have multi-dose preventative properties. After an overdose has occurred, the situation is usually dealt with by first responders and hospitals using antidotes or medical procedures to limit the absorption of the drug. As pharmaceutical scientists, therein lies an opportunity to produce novel formulations that could limit the chances of accidental overdose. One approach could be to harness the physiological properties within the gastrointestinal tract (GIT), especially the enzymatic degradation of macromolecular matrix formulations. The ideal formulation will deliver a therapeutic dose but prevent or limit further release from consequent dose forms if a toxic quantity of drug is consumed.
Download the full article as PDF (“pre-proof”) here Existing and emerging mitigation strategies for the prevention of accidental overdose from oral pharmaceutical products Review
or read it here
Example from the publication: Multi-dose abuse-preventive formulations
In recent years there has been some research reported exploring multi-dose abuse-preventive formulations. These formulations propose techniques which could stop the release of drug when multiple dosages are consumed. One approach designed by the Patel group utilises pH sensitive Eudragit® polymers to prevent release of drug when multiple doses were taken together. Figure 4 shows the concept behind a loperamide multi-dose abuse formulation [45]. The drug was hot melt extruded with gastric soluble polymers (Eudragit® EPO or Kollicoat® Smartseal 100P) and a base (L-arginine). The researchers hypothesised that if sufficient base was present when a dangerous quantity (15 tablets in their experiment) was consumed by co-lease with the initial amount of drug, then the pH of the surrounding gastric solution would increase to > pH 5, thus preventing further dissolution of the polymer and hence prevent further release of drug. Results indicated that when one dose was exposed to gastrointestinal fluid, release of drug equivalent to a therapeutic dose was achieved, but when multiple doses were taken only 2% of drug was released compared to the current marketed product Imodium®.
Mubtasim Murshed, Malinda Salim, Ben J. Boyd, Existing and emerging mitigation strategies for the prevention of accidental overdose from oral pharmaceutical products Review, European Journal of Pharmaceutics and Biopharmaceutics, 2022, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2022.10.002

