Nose-to Brain Delivery of Resveratrol, a Non-Invasive Method for the Treatment of Cerebral Ischemia
Abstract
Cerebral ischemia represents a particular condition among neurological diseases due to its high frequency, high associated mortality, and the permanent disability in patients that survive it. Numerous studies in animal models have demonstrated the protective properties of resveratrol against cerebral ischemia. Resveratrol is a soluble molecule in polar solvents with high membrane permeability; however, it is rapidly metabolized at the liver and is also a substrate of the ATP binding cassette transporters located at the blood–brain barrier. These circumstances reduced bioavailability of resveratrol to the brain. In this review, we examined nasal resveratrol’s formulations including nanocarriers such as nanostructured lipid carriers, nanoemulsions, nanoparticles, bilosomes, cubosomal, and transferosomes that are directly transported to the brain. An intranasal administration route evades resveratrol transformation due to liver metabolism. Components of nanoformulations increased resveratrol absorption to the brain by enhancing permeation through specific approaches and also maintaining stability during storage. Both characteristics improved the delivery of resveratrol with conserved antioxidant capacity and protective properties for neurological models. Although demonstration that the nanoformulations prevents resveratrol’s blood–brain barrier retention is missing, properties of resveratrol’s nanoformulation encourage testing in clinical trials; however, regulatory approval for a novel nanocarrier in nasal drug delivery is complicated and needs approval.
Introduction
Resveratrol (3, 5, 40-trihydroxystilbene) is a natural polyphenol produced in many plants (e.g., grapes, peanuts, and hops) as a defense molecule against microbial infections and physical injury [1] (Figure 1). In plants, the content of resveratrol is limited, therefore, to achieve commercial purposes, it has been obtained from Japanese knotweed and grape skins and seeds, which has been reported to have a high content (ranging from 2 to 13 mg/L). Also, due to resveratrol’s multiple pharmacological activities found in humans, alternative methods to obtain larger amounts of resveratrol have been implemented, like chemical synthesis and biotechnological approaches, such as tissue culture and genetic engineering [2].
Table 1. Nanotechnology-based formulations for resveratrol delivery.
| Drug Delivery Nanosystem | Structural Characteristics | Drug Encapsulation Efficiency (%) | Spherical Shape (nm) | Observations |
|---|---|---|---|---|
| Vesicles | ||||
| Gelot 64 (SF) oleic or linoleic acids (ST) | Resveratrol 35 ± 2 | 299–402 | Ex vivo skin permeation experiments indicated that niosomes prepared with the ethanol injection are more effective. | |
| NIOSOMES Formed by an aqueous core enclosed within a non-ionic surfactant bilayer. | Span 60 (SF) Dodecanol (ST) | Resveratrol 64 ± 8 | 168 ± 4 | Niosomes exhibited a small mean size, narrow size distribution, high entrapment efficiency, and good stability |
| Maisine 35–1 (SF) Dodecanol (ST) | Resveratrol 53 ± 8 | 175 ± 13 | Niosomes exhibited a small mean size, narrow size distribution, high encapsulation efficiency and good stability | |
| Span 60 (SF) Cetyl alcoho (ST) | Resveratrol 81 ± 2 | 108 ± 3 | Nanoencapsulation of resveratrol using non-ionic surfactant and fatty alcohol dramatically improved its solubility and functionality | |
| OramixTM CG110 (SF) LauroglycolTM FCC (SF) Soybean phosphatidylcholine (ST) Oleico acid (ST) | Resveratrol >70 | 90 | Toxicity of the vesicular formulations evaluated in vitro, showed no alteration on cell viability after incubation with RSV loaded vesicles. | |
| LIPOSOMES Formed by phospholipids, which can include several other constituents like cholesterol to improve the stability of the bilayer. | Soybean lecithin (ST) Oleico acid (ST) N-succinyl chitosan as protective shell of liposomes | Resveratrol and Quercetin 70 ± 5 | 82 ± 3 | The succinyl-chitosan shell increased physical stability of the vesicular system and release of the polyphenols to a greater extent at pH 7.0 (mimicking the intestinal environment). |
| Tri-block polymer L64 D-α-tocopheryl polyethylene glycol 1000 succinate (SF) Phospholipon 90G (ST) | Resveratrol 95 | 86 ± 3 | Biocompatibility was demonstrated in an ex vivo model of hemolysis in human erythrocytes. Liposomes maintained the antioxidant properties of resveratrol. | |
| Emulsions | ||||
| Soybean oil (OP) Soy lecithinas (SF) Labrasol (ST) | Resveratrol 92.5 ± 2.2 | 132 ± 9 | The improved oral bioavailability of resveratrol was attributable to the inhibition of intestinal glucuronidation by the presence of labrasol. | |
| Labrafac (OP) Cremophor® 40 (SF) Hyaluronic acid (SF) | Resveratrol and curcumin N. A | 115.2 ± 0.2 | The mucoadhesive nanoemulsion was safe to nasal mucosa and increase amount of both polypehnols in the brain. | |
| Soybean phospholipiddipropylene glycol (OP) 2-hydroxypropyl-β-cyclodextrin (SF) Phosphatidyl choline 60 (SF) | Resveratrol N. A | 122 ± 08 | Nanoformulation presented good compatibility and skin permeation. | |
| Olive oil (OP) Polyethylenglycol Pluronic® P105 (SF) Cremophor® EL (SF) | Resveratrol N. A | 155 ± 33 | Perfluoropolyether was used as a nano emulsion tracer. Resveratrol-loaded nano emulsions was internalized by macrophages | |
| Capryol™ 90 (OP) Cremophor® EL (SF) Tween 20 (SF) | Resveratrol N. A | 41.3 ± 4 | The formulation increased oral bioavailability 3.2-fold compared to resveratrol alone. Anti-fatigue properties of resveratrol were improved. | |
| SIMPLE EMULSIONS Formed by emulsifiers dispersed in oil, and water. | Labrafil (OP) Gelucire® 44–14 (SF) Labrasol (SF) | Resveratrol N. A | 21 ± 5 | Nanoemulsions significantly increased intestinal permeation across rat jejunum. |
| Labrafil (OP) Miglyol® 812 (SF) Tween 80 (SF) | Resveratrol N. A | 103 ± 14 | Nanoemulsion significantly increased intestinal permeation across rat jejunum. | |
| Labrafil (OP) Labrasol (SF) Cremophor RH 40 (SF) | Resveratrol N. A | 26 ± 1 | Formulation components inhibited the UDP-glucuronosyltransferase and consequently increases the bioavailability of resveratrol. | |
| Miglyol® 812 (OP) orange oil (OP) Quinoa starch particles (SF) Octenyl succinic anhydride (SF) | Resveratrol 98 ± 2 | 50,000 | Appropriate resveratrol carrier system for use in oral formulations. | |
| Miglyol® 812 (OP) orange oil (OP) Quinoa starch particles (SF) Tween 20 (SF) | Resveratrol 63 ± 1 | 50,000 | Appropriate resveratrol carrier system for use in oral formulations. | |
| Olive oil (OP) Sodium lignin sulfonate (SF) Tween 80 (SF) PEG-400 (SF) | Resveratrol N. A | 119 ± 5 | Excellent biocompatibility, improved solubility, and showed antiradical efficiency compared to free trans-RSV | |
| Medium-chain Triacylglycerols (OP) Tween 80 (SF) For double layer nanoemulsions: chitosan, alginate or β-cyclodextrin | Resveratrol N. A | 27 ± 55 | The stability of resveratrol in the double-layer nanoemulsions complexed with chitosan or β-cyclodextrin was higher, compared with single-layer nanoemulsions. | |
| Miglyol® 812 (OP) The inner aqueous phase: ethanol, RSV, and 0.1 M NaCl solution The external aqueous phase: carboxymethylcellulose, Tween 20, and 0.1 M NaCl. | Resveratrol 55 | 59,800 ± 0.002 | High encapsulation efficiency and slow storage release. | |
| MULTIPLE EMULSIONS Formed by multiple emulsions of water-in-oil-in-water. | Single-layer emulsion: lactoferrin Multilayer emulsion: lactoferrin/alginate lactoferrin/alginate/ε-poly-L-lysine | Resveratrol N. A | 249 | The antioxidant activity of resveratrol-loaded emulsion did not significantly change during storage, whereas it decreased in nonencapsulated resveratrol oil from the third week onwards. |
| The single layer: ethanol/water RSV solution Miglyol 812 Polyglycerol polyricinoleate (ST) Multilayer emulsion: Tween 20 solution with and without sodium carboximethylcellulose as thickening agent. | Resveratrol 77.50 | 10,000–30,000 | The emulsion showed high encapsulation efficiency, good storage stability, shear-thinning behavior, and dominant elastic character. These double emulsions may be suitable for food applications. |
|
| Particles | ||||
| LIPIDIC NANOPARTICLES Formed by a solid hydrophobic core stabilized by a hydrophilic layer of surfactant molecules or polymers. | Tristearin (OP) Chitosan (ST) Hydrogenated phosphatidylcholine (SF) | Resveratrol 67 | 6000 | The cream containing the chitosan-coated lipidic nano particles produced a significant enhancement in the in vivo permeation of resveratrol |
Stroke is placed as the second leading cause of global death, with a high number of survivors being permanently incapacitated. Interestingly, the statistics have been changing: stroke was considered a disease of the developed world, nevertheless, the burden of stroke seems to be shifting to the developing countries, somewhat due to poor community information of stroke notice signs (which is the main cause of delay in arrival to the hospital). A wide range of stroke risk factors have been recognizably responsible for 90% of all strokes, among them, age is the strongest determinant, since age doubles the risk of stroke every decade above the age of 55 years. Regrettably, individuals who survive stroke live with a profound effect on health-related quality of life [3]. Generally, stroke is classified as an ischemic stroke, caused by interruption of the blood supply to a specific region of the brain (the most common type) and hemorrhagic stroke, attributed to the rupture of a blood vessel. In an ischemic stroke, the reduction of the steady blood flow provokes hypoxia and hypoglycemia that leads to severe affection to brain cell function. Typical treatment for ischemic stroke is the restoration of blood flow/oxygenation to the brain in a timely fashion by thrombolysis, using the intravenous recombinant tissue-type plasminogen activator, or by mechanical thrombectomy [4]. Paradoxically, reperfusion therapy after a prolong period of ischemia (>3 h) often leads to further cerebral damage. Ischemia and reperfusion activates a cascade of events including excitotoxicity, driven by the excessive activation of N-methyl-D-Aspartate glutamate receptors; oxidative stress, associated with mitochondrial dysfunction and the increase of reactive oxygen species production; the inflammatory response, which can cause damage due to high concentrations of inflammatory mediators; autophagy, a type of cell death induced by organelle damage and extracellular injury stimulation; and the blood–brain barrier (BBB) breakdown process, initiated by the deterioration of thigh junction proteins at the endothelial cells and aggravated by inflammation [5,6,7].
Unfortunately, establishing an effective treatment for stroke has been unfavorable; therefore, it is indispensable to continue the study of compounds that alleviate the damage induced in animal models in future clinical studies.
The ineffectiveness of pharmacological therapies in the clinical setting may stem from the omission of significant comorbidities, such as aging, in experimental models. Notably, the limitations of drugs exhibiting protective effects in the preclinical phase involve issues of bioavailability and challenges in crossing the BBB. Considering that the majority of strokes occur in elderly patients [8], it is crucial to recognize that drug absorption in the brain undergoes age-related changes. For instance, a key transport mechanism frequently employed for drug delivery to the brain, transcytosis through the transferrin receptor, is compromised during the aging process [9]. Additionally, in murine models of aging, an observed increase in certain efflux transporters poses a significant obstacle to the efficient delivery of drugs to the brain [10]. Not surprisingly, the combination of these mechanisms leads to dramatically low net delivery fluxes of resveratrol to the brain. This is particularly relevant when attempting to reverse brain damage following cerebral infarction in patients undergoing aging processes.
Table 2. Formulations for resveratrol intranasal delivery to the brain.
| Formulation | Components | Physical Characteristics | EE (%) PDI ZP (mV) | Observations |
|---|---|---|---|---|
| Vitamin E nanoemulsion | Vitamin E:sefsol (1:1) (ST) Tween 80 (SF) Transcutol P (co-SF) | Transparent and monophasic. Spherical globules (102 ± 1.46 nm). | N.D. 0.158 ± 0.02 −35 ± 0.02 | Histological studies showed decreased degenerative changes |
| F6SPION/W3 SPION-loaded with chitosan coated bilosomes | Cholesterol:sorbitan monosterate (Span 60) (1:1), sodium deoxycholate (10 mg), glycerin. Chitosan (0.1%). SPION (magnetite, Fe3O4, 10.3 mg/mL). Waffers: sodium alginate: poly vinyl pyrrolidone K-25 (1:2) + glycerol (10%). | Black spheres surrounded by a layer of less intense black color (chitosan coating layer). Porosity 88% (243 ± 1.87 nm). | 85 ± 1.08 0.14 ± 0.06 35 ± 2.05 | Resveratrol (20 mg/Kg/day, i.n.) improved induced memory and cognitive functions in mice with induced Alzheimer model with lipopolysaccharide. |
| Cubosomal in situ nasal gel | 4% w/v glicerol monooleate and 1.5% Lutrol F127. 12% w/v Poloxamer 407 (cubosomal gel, permeation enhancer) | Cubical in shape, uniform with smooth surface (161.5 ± 0.12 nm). | 83.08 ± 0.21 0.279 ± 0.15 −20.9 ± 0.11 | The formulation showed higher trans-nasal permeation and brain’s distribution compared to resveratrol solution. |
| Transferosomes capped with gold nanoparticles [gold (III) chloride HauCl4] | Soy lecithin (glycerophopholipids), ethanol (permeation enhancers), and Cremophor RH 40 (SF) in a ratio of 46.7:20:33.3 (w/w%). | Gold- nanoparticles (GNP), resveratrol-transferosomes-GNP, and resveratrol-nanoemulsion-GNP showed uniform spherical shape with size of 10.30 + 2.4, 94.93 + 5.6, and 20.36 + 4.3 nm | 69.53 ± 3.82 0.194 ± 0.08 −28.7 ± 4.7 | Transferosomes penetrated through nasal mucosal layers. Resveratrol-trans-GNP gel enhanced the spatial memory recovery in amnesic rats compared to i.n. pure resveratrol. |
| Nanoemulsion capped with gold nanoparticles [gold (III) chloride HAuCl4] | Capryol 90 (ST) Tween 20 (SF), and transcutol (co-SF) at a ratio of 30:60:10 v/v%. | Gold- nanoparticles (GNP), resveratrol-transferosomes-GNP, and resveratrol-nanoemulsion-GNP showed uniform spherical shape with size of 10.30 + 2.4, 94.93 + 5.6, and 20.36 + 4.3 nm | 95.72 ± 5.34 0.26 ± 0.04 −18 ± 2.6 | Transferosomes penetrated through nasal mucosal layers. Resveratrol-trans-GNP gel enhanced the spatial memory recovery in amnesic rats compared to i.n. pure resveratrol. |
| Nonoestructured lipid carrier loaded in a situ gel | Cetyl palmitate (solid lipid), Capmul MCM (ST) 1:1 ratio. Acrysol (nonionic, solvent-free and hydrophobically modified ethylene). Poloxamer 188 (SF), Tween 80 (SF). Gellan gum 0.5% and xanthan gum 0.15% (in situ gel formulation) | 132 ± 11.9 nm | 74.05 ± 11.40 0.209 ± 0.005 −23 ± 3.79 | The scopolamine-induced amnesia was reduced with the optimized formulation. |
| Chitosan-coated lipid microparticles | Phosphatidylcholine (SF) 1.75%, w/v, Stearic acid (ST) Chitosan (bioadhesive excipient) | 68.5 ± 3.1 µm | N.D. N.D. −12.7 ± 2.1 | Resveratrol was found in the rat cerebrospinal fluid with no distribution in blood after nasal administration. |
| Chitosan-coated lipid microparticles | Phosphatidylcholine (SF) 1.75%, w/v, Stearic acid (ST) Chitosan (bioadhesive excipient) | 76.3 ± 5.2 µm | 76.50% N.D. 24.0 ± 4.7 | Resveratrol was found in the rat cerebrospinal fluid with no distribution in blood after nasal administration. |
| Chitosan-coated lipid microparticles | Phosphatidylcholine (SF) 1.75%, w/v, Stearic acid (ST) Chitosan (bioadhesive excipient) | 84.5 ± 8.1 µm | 81.00% N.D. 44.6 ± 3.1 | Resveratrol was found in the rat cerebrospinal fluid with no distribution in blood after nasal administration. |
| Ionic-sensitive in situ gel loaded with resveratrol nanosuspensions | 0.6% w/v gellan gum | Spherical shape in gellan gum matrix and dispersed uniformly without adhesions. | N.D. 0.234 −8.8 | The increase in the bioavailability of resveratrol in the brain showed in the pharmacokinetics studies suggested the direct nose-to brain delivery. |
| Nanosized transferosome based intranasal in situ gel | Transferosome Soya lecithin/PE (ratio 7:3) + (Cremophor RH 40 (SF) Gel: Poloxamer 407 (18%) and Carbopol 934 (0.4%) | Spherical shape (83.79 ± 2.54 nm) | 72.58 ± 4.51% N.D. N.D. | Resveratrol bioavailability was enhanced through the nasal route of administration. |
| Hyaluronic acid based lipidic nanoemulsion | Labrafac Lipophile/cremophor 40 10% (SF); hyaluronic acid 1.5% w/v | Spherical morphology and nanometer size (115 ± 0.15 nm). | N.D. 0.235 ± 0.01 −23.9 ±1.7 | The pharmacokinetic analysis showed that the intranasally administered mucoadhesive formula allowed the absorption of resveratrol and curcumin reaching the brain. |
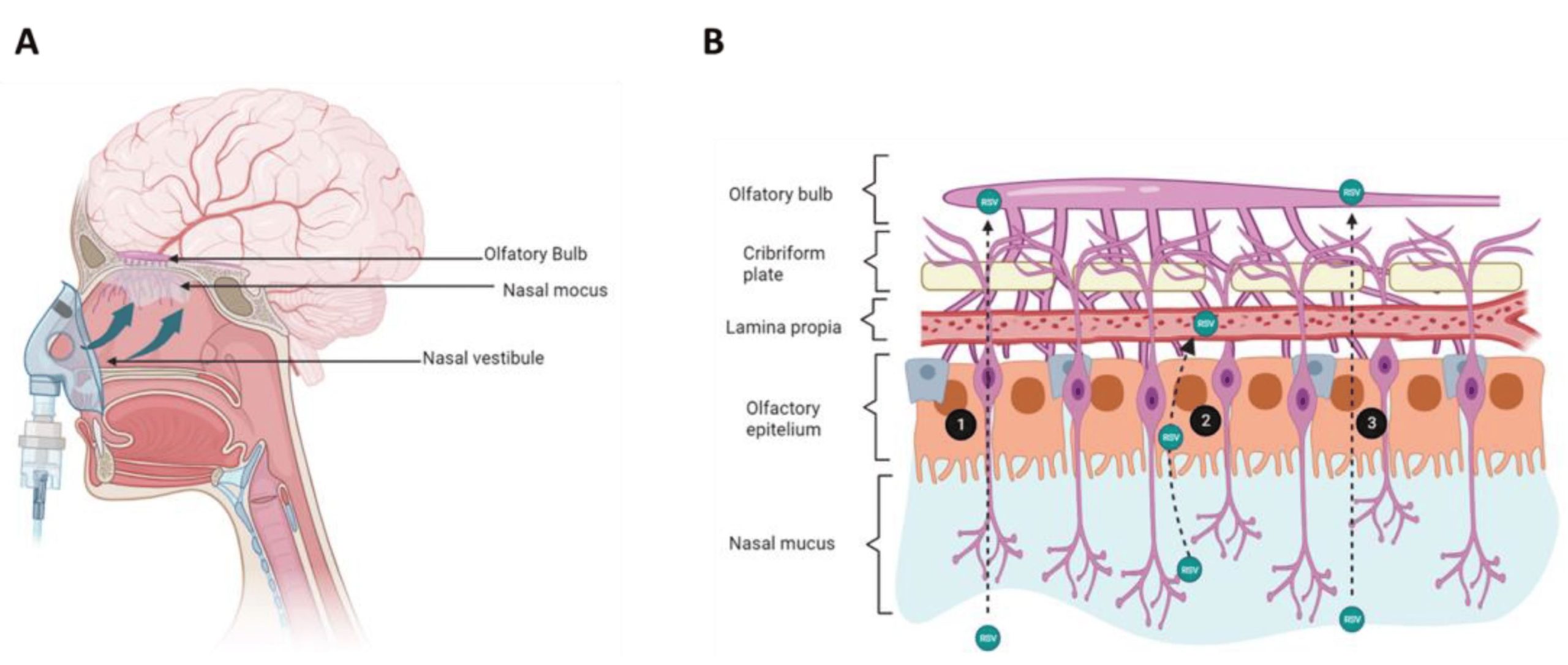
Therefore, it is imperative to develop a biocompatible formulation that operates autonomously from the expression of endogenous transport systems and can effectively traverse natural barriers, even in the presence of aging processes. For that reason, the design of intranasal formulations of materials that fusion with membranes is currently seen as a promissory alternative for its use in clinical studies; this route allows direct transport to the brain from the nasal cavity along the olfactory and trigeminal nerves, hence, it is a critical step that will facilitate the delivery of the drugs to the brain.
Download the full article as PDF here Nose-to Brain Delivery of Resveratrol, a Non-Invasive Method for the Treatment of Cerebral Ischemia
or read it here
Alquisiras-Burgos, I.; González-Herrera, I.G.; Alcalá-Alcalá, S.; Aguilera, P. Nose-to Brain Delivery of Resveratrol, a Non-Invasive Method for the Treatment of Cerebral Ischemia. Drugs Drug Candidates 2024, 3, 102-125. https://doi.org/10.3390/ddc3010007
See also the interesting video on Vitamin E TPGS below and read more here:

