Drug‑Excipient Compatibility Study Through a Novel Vial‑in‑Vial Experimental Setup: A Benchmark Study
Abstract
Drug-excipient compatibility study (DECS) is one of the critical steps during pre-formulation studies to select the appropriate excipient to obtain a stable formulation/dosage form. As such, there is no recommended guideline for DECS. Further, the previously reported studies and protocols followed by various pharmaceutical industries are very lengthy and laborious. Therefore, to improve the existing study strategies and rapid screening of suitable excipients during formulation development, a novel vial-in-vial approach has been proposed. The devised approach was compared with the previously reported conventional approaches using six different drugs with multiple marketed formulations from different manufacturers for each drug.
To validate the proposed novel approach, several reported strategies/methodologies have been executed such as exposure of formulations with and without primary packaging, crushed blend with and without water, and/or acetonitrile at accelerated stability condition of 40°C/75% RH for 3 to 6 months and compared with the novel approach. Eventually, all the samples were subjected to HPLC analysis to evaluate the degradation behaviour. The results suggested that the novel approach demonstrated discriminating results with significant degradation as compared to the conventional approaches. Consequently, exercising this methodology for screening the excipients is expected to shorten the drug development cycle by many folds. Moreover, it has also been anticipated that the developed novel approach would prevent the occurrence of late-stage surprises during stability studies.
Introduction
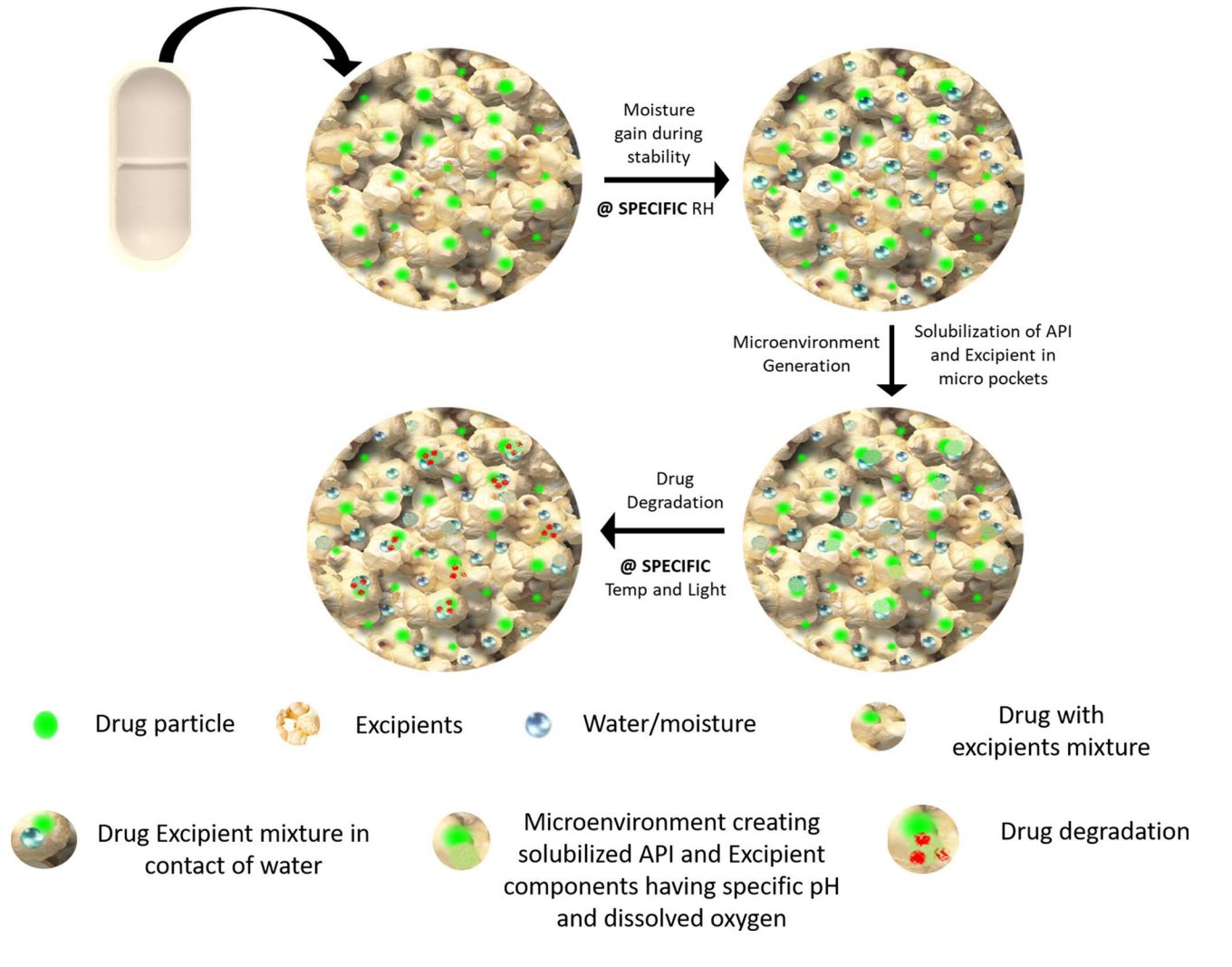
Soon after winning the race for investigational new drug approval, the next step is to develop a stable formulation for clinical supply with the most suitable excipients[1]. Despite being recognized as inert, excipients can have a substantial impact on drug stability as they can interact with drug substances. The reason for chemical reactions/ degradation of the drug includes adsorption of water during storage, generation of microenvironmental pH, and activation of functional groups present in excipients/impurities of excipients in the presence of light, heat, and moisture during storage. For instance, the excipients containing reducing sugars (e.g. lactose, maltose, and glucose) or the presence of reducing sugar as an impurity in excipients such as microcrystalline cellulose can promote Maillard reaction in primary and secondary amine-containing drugs[2–7]. Peroxides or phenolic impurities present in povidone and crospovidone can lead to the oxidation or photodegradation of the drug[8, 9]. There are many reports on the observation of aldehydes in lactose, MCC, starch, PEG, etc. that can also cause drug degradation[10, 11]. Therefore, drug-excipient compatibility study (DECS) is being carried out at the pre-formulation stage to select the pertinent excipients for formulation development. The DECS is not only an important but also an obligatory part of regulatory submission. All abbreviated new drug application submission requires a detailed DECS as a par of generic formulation development, where usually the excipient used in generic formulations is diferent from the innovator formulation. According to ICH Q8, it is also a mandatory requirement to assess the compatibility of Active Pharmaceutical Ingredients (API) with all excipients included in the formulation development[12–14].
Industry 4.0 is growing swiftly across the board, and any excess time spent on product development has a signifcant negative economic infuence on the industry’s sustainability. The traditional DECS is a labor-intensive and tedious procedure, where a binary or multi-component physical mixture of drugs and excipients is prepared and charged on stability at accelerated conditions (with or without water) for an extended period of time. Further, the samples are analyzed at a pre-specifed time point, usually 1 to 3 months[15, 16]. Hence, there is a constant need for an efective and reliable approach that can screen excipients rapidly.
The studies between the interaction of API and excipients have been the subject of discussion among scientists for decades and a few approaches have been put forward to save time and expenses[8]. A DECS methodology adopted by Serajuddin et al. involves adding 20% water to the drug-excipient blend and charging it at 50°C[17]. However, in this study, additional water has been added to each sample, which shall not always be the case in actual oral solid formulations. Further, excessively added water might force hydrolysis even if the excipients used are non-hygroscopic. In another system proposed by Sims et al., the drug-excipient blend was stressed to 100°C and 80°C in the specially designed vial and block system[18]. The temperature applied for the study was very extreme, which can produce unrealistic degradation products (DPs) of the drug substance. In an attempt to perform high-throughput screening for DECS, Wyttenbach et al. placed 10–20 mg excipient to each well of 96-well plate, followed by the addition of 20 µL of drug solution prepared at the concentration of 5 mg/mL in ethanol[19]. The samples were charged at 40°C or 50°C and 10% or 75% RH. Most of the BCS class II and IV molecules have poor water solubility to achieve 5 mg/mL concentration. Moreover, the solvent other than water may lead to a very diferent/non-realistic environment for the excipient to behave during stability conditions. None of the above-reported techniques ofers realistic and discriminative opportunities for excipients to produce a suitable microenvironment for the drug. Several other methods based on diferential thermal calorimetry, thermogravimetry, Raman spectroscopy, FTIR spectroscopy, and the XRPD principle are also reported in the literature[20–23]. Although the above techniques have the advantage of being rapid with low sample size requirements, they do have the limitation of complex data interpretation. Furthermore, the type of degradation occurred cannot be identifed, and most importantly, the role of microenvironmental pH on the degradation of drugs cannot be assessed.
The endeavor of the current study is to propose a unique approach for DECS where a balance between time and realistic conditions can be achieved. Moreover, the target procedural attribute was to allow the drug-excipient(s) mixture to absorb moisture based on their physicochemical characteristics and have a chemical interaction. The proposed approach has been validated with six diferent drugs and compared with six other traditional/reported approaches.
Download the article as PDF here Drug‑Excipient Compatibility Study Through a Novel Vial‑in‑Vial Experimental Setup: A Benchmark Study
or read it here
Drug‑Excipient Compatibility Study Through a Novel Vial‑in‑Vial Experimental Setup: A Benchmark Study, Sonali Jain, Ravi P. Shah, Received: 23 March 2023 / Accepted: 19 April 2023 / Published online: 10 May 2023, © The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists 2023, AAPS PharmSciTech (2023) 24:117, https://doi.org/10.1208/s12249-023-02573-0
Read more on Introduction of Shellac as a pharmaceutical excipient here:


