Formulation and Development of Paediatric Orally Disintegrating Carbamazepine Tablets
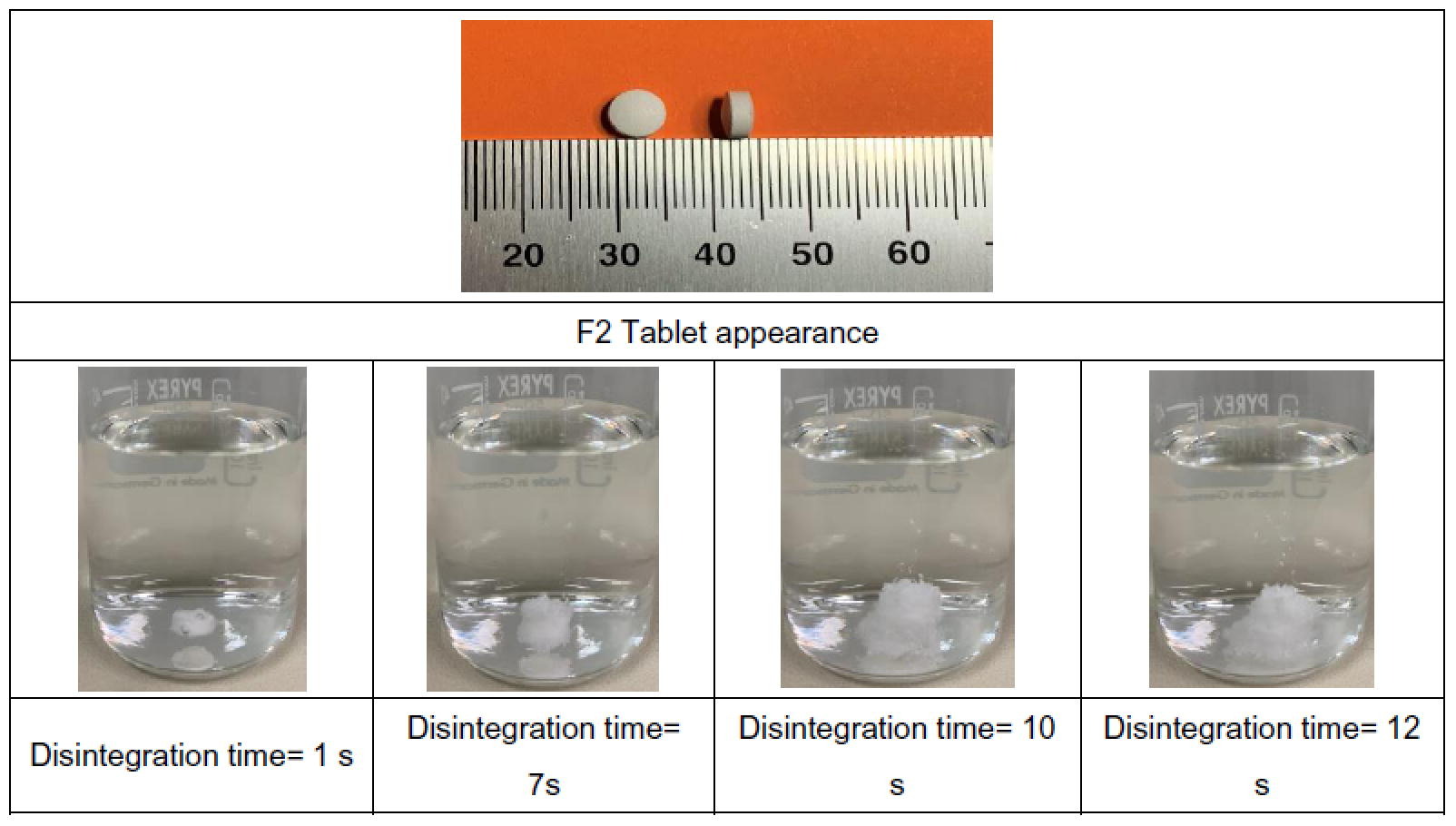
Carbamazepine is a medicine used to manage epilepsy and partial or tonic-clonic seizures. This study aimed at formulating and obtaining carbamazepine orodispersible tablets for paediatric use at a 50 mg dose, with a diameter not greater than 6 mm and a tablet weight of 80 mg, through a direct compression process. The SeDeM pre-formulation/formulation method was used to define the characteristics of both carbamazepine and the selected excipients for direct compression. This study succeeded in formulating and obtaining the proposed tablets. Following the application of the SeDeM method, the tablets met the mass uniformity test and showed appropriate hardness values for orodispersible tablets. The tablets also met the United States Pharmacopeia (USP) test specifications at t = 60 min. The orodispersible tablets obtained may improve compliance with paediatric treatment with carbamazepine, ensuring the safety and effectiveness of the medicine.
Download the full study as PDF here Formulation and Development of Paediatric Orally Disintegrating Carbamazepine Tablets
or read it here
Materials
The active pharmaceutical ingredient (API) used was carbamazepine (batches: 16CT000089 and 16CT000017), obtained from CTX Lifesciences Pvt. Limited, India.
The main excipients used were: L-HPC LH11 [(low-substituted hydroxypropylcellulose) (supplier: SHINETSU, Tokyo, Japan)]; L-HPC NBD022 [(low substituted hydroxypropyl cellulose) (supplier: SHINETSU, Tokyo, Japan)].
The adjuvant excipients used were: VIVASOL GF® [(croscarmellose) (batch: 7111601027, supplier: JRS PHARMA, Rosenberg, Germany)]; VIVAPHARM PVP PXL® [(crospovidone type A) (supplier: JRS PHARMA, Rosenberg, Germany)]; VIVAPHARM PVP PXL10® [(Crospovidone Type B) (supplier: JRS PHARMA, Rosenberg, Germany)]; PEARLITOL 200 SD® [(D-Mannitol) (supplier: ROQUETTE LAISA ESPAÑA, Valencia, Spain)]. The excipients used as lubricants and ligants were: Talc (supplier: Fagron, Barcelona, Spain); magnesium stearate (supplier: Fagron, Barcelona, Spain) and colloidal silicon dioxide (Aerosil®) (supplier: Fagron, Barcelona, Spain) as a standard lubrication blend. All components were stored at room temperature (20-30°C).
R Canadell-Heredia, M Suñé-Pou, A Nardi-Ricart, P Pérez-Lozano, JM Suñé-Negre, E García-Montoya, Formulation and Development of Paediatric Orally Disintegrating Carbamazepine Tablets, Saudi Pharmaceutical Journal, 2022, ISSN 1319-0164,
https://doi.org/10.1016/j.jsps.2022.09.004.

