Formulation development, optimization and characterization of Pemigatinib-loaded supersaturable self-nanoemulsifying drug delivery systems
Background
Pemigatinib is a small molecule tyrosine kinase inhibitor of fibroblast growth factor receptor inhibitors. The oral bioavailability of Pemigatinib is constricted due to its limited solubility at physiological pH. It is essential to develop a novel formulation of Pemigatinib to improve the intrinsic solubility and to reduce the pharmacokinetic variability. Self-nanoemulsifying drug delivery system is an effective, smart and more adequate formulation approach for poorly soluble drugs. Different from conventional self-nanoemulsifying drug delivery system, a supersaturable self-nanoemulsifying drug delivery system of Pemigatinib was prepared by using a supersaturation promoter.
Results
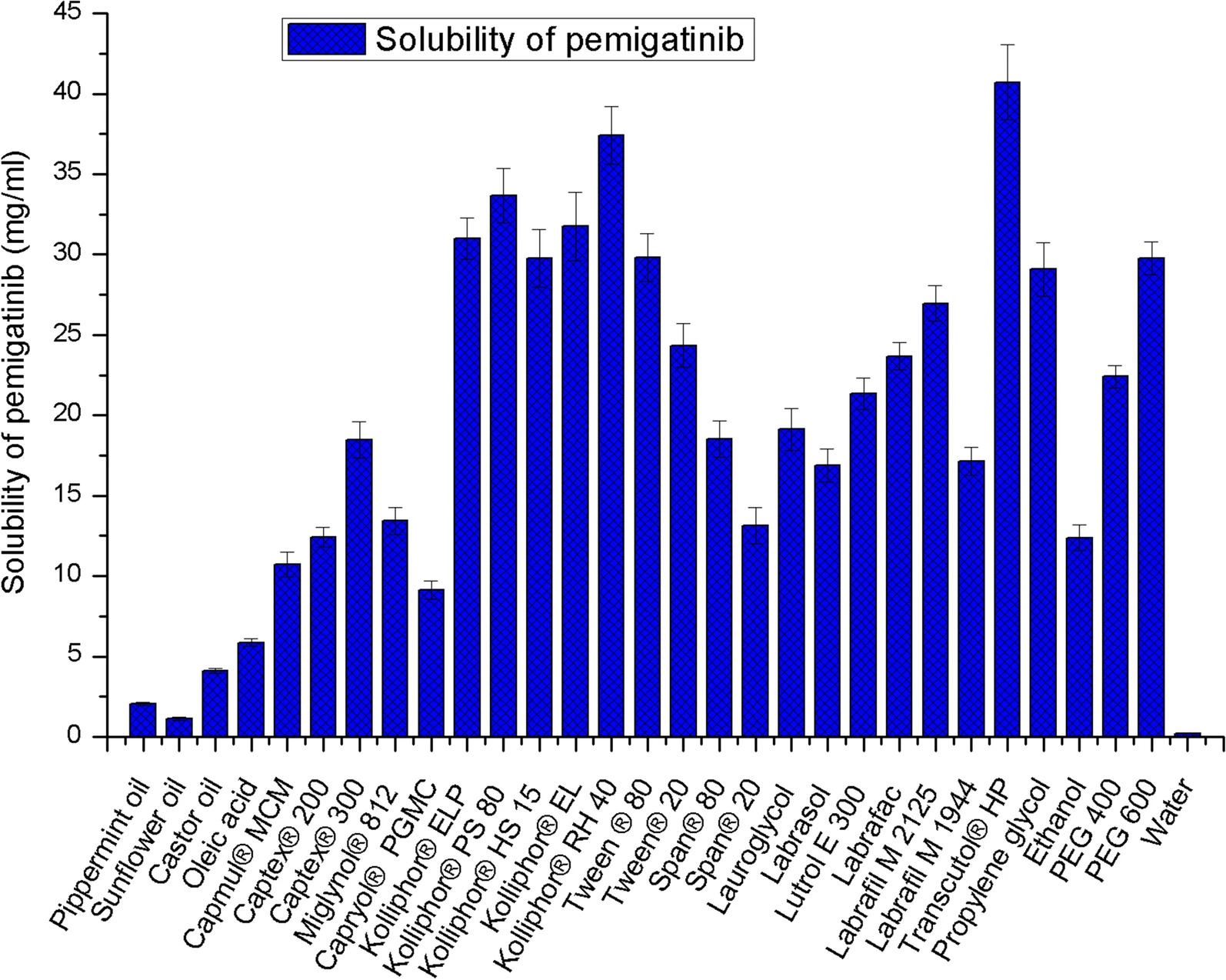
Among all the oils, Captex® 300 have shown maximum solubility of Pemigatinib. Considering the solubilization potential and emulsification ability Kolliphor®RH 40 was selected as surfactant. Transcutol®HP was selected as co-surfactant. The composition of oil, surfactant and co-surfactant was identified using phase diagrams and further adjusted by simplex-lattice design. HPMC K4M as precipitation inhibitor at 5% concentration resulted in effective supersaturating with increased self-emulsification time. The droplet of sSNEDDS ranges from 166.78 ± 3.14 to 178.86 ± 1.24 nm with PDI 0.212 – 0.256, which is significantly smaller than that observed with plain SNEDDS. TEM images revealed the spherical shape of the nanodroplets. The final optimized formulation formed spontaneous nanoemulsion within 15 secs when added to physiological fluids. The percent transmittance of the diluted formulation was found to be 99.12 ± 0.46. The viscosity was found to be 574 ± 26 centipoises indicating the good flow ability. FTIR and DSC studies indicated the amorphization of the drug. The dissolution profile of sSNEDDS indicated the faster release of drug compared to both pure drug suspension and SNEDDS formulation. The drug release rate is directly proportional to the concentration of the drug. The drug release from the insoluble matrix is a square root of time-dependent Fickian diffusion process. The formulation was found to be stable and transparent at all pH values and the percent transmittance was more than 95%. Any kind of separation or precipitation was not observed at different temperatures cycles. No significant difference was observed with all the samples exposed at different storage conditions.
Conclusions
This study demonstrated the feasibility of stabilizing and improving the in-vitro performance of self-nanoemulsifying drug delivery systems of Pemigatinib by incorporating HPMC K4M as precipitation inhibitor.
Download the full study as PDF here Formulation development, optimization and characterization of Pemigatinib-loaded supersaturable self-nanoemulsifying drug delivery systems
or read it here
Materials
Pemigatinib was procured from Aelida Pharmaceuticals, Haryana, India. Sunflower oil, Peppermint oil, Oleic acid, Castor oil, Capmul®MCM, Captex®300, Captex®2000, Miglynol®812 and Capryol®PGMC were purchased from HI Media Private limited, Mumbai, India. Tween®80, Tween®20, Span®80, Span®20, PEG 600, PEG 400, Propylene, Acetonitrile, Ethanol and Methanol were procured from SD fine chemicals limited, Mumbai, India. Kolliphor®HS15, Kolliphor®PS80, Kolliphor®ELP, Kolliphor®EL, Kolliphor®RH40 were obtained from BASF, Germany. Lauroglycol, Labrasol, Lutrol E 300, Labrafac, Labrafil M 2125, Labrafil M 1944 were obtained from Loba Chemie Private Limited, Mumbai, India.
Reddy, M.R., Gubbiyappa, K.S. Formulation development, optimization and characterization of Pemigatinib-loaded supersaturable self-nanoemulsifying drug delivery systems. Futur J Pharm Sci 8, 45 (2022).
https://doi.org/10.1186/s43094-022-00434-4

