Effect of different seed crystals on the supersaturation state of ritonavir tablets prepared by hot-melt extrusion
Hot-melt extrusion (HME) is a technology increasingly common for the commercial production of pharmaceutical amorphous solid dispersions (ASDs), especially for poorly water-soluble active pharmaceutical ingredients (APIs). However, recrystallization of the APIs during dissolution must be prevented to maintain the supersaturation state enabled by ASD. Unfortunately, the amorphous formulation may be contaminated by seed crystals during the HME manufacturing process, which could lead to undesirable crystal growth during the dissolution process. In this study, the dissolution behavior of ritonavir ASD tablets prepared using both Form I and Form II polymorphs was examined, and the effects of different seed crystals on crystal growth rates were investigated. The aim was to understand how the presence of seed crystals can impact the dissolution of ritonavir, and to determine the optimal polymorph and seeding conditions for the production of ASDs.
Highlights
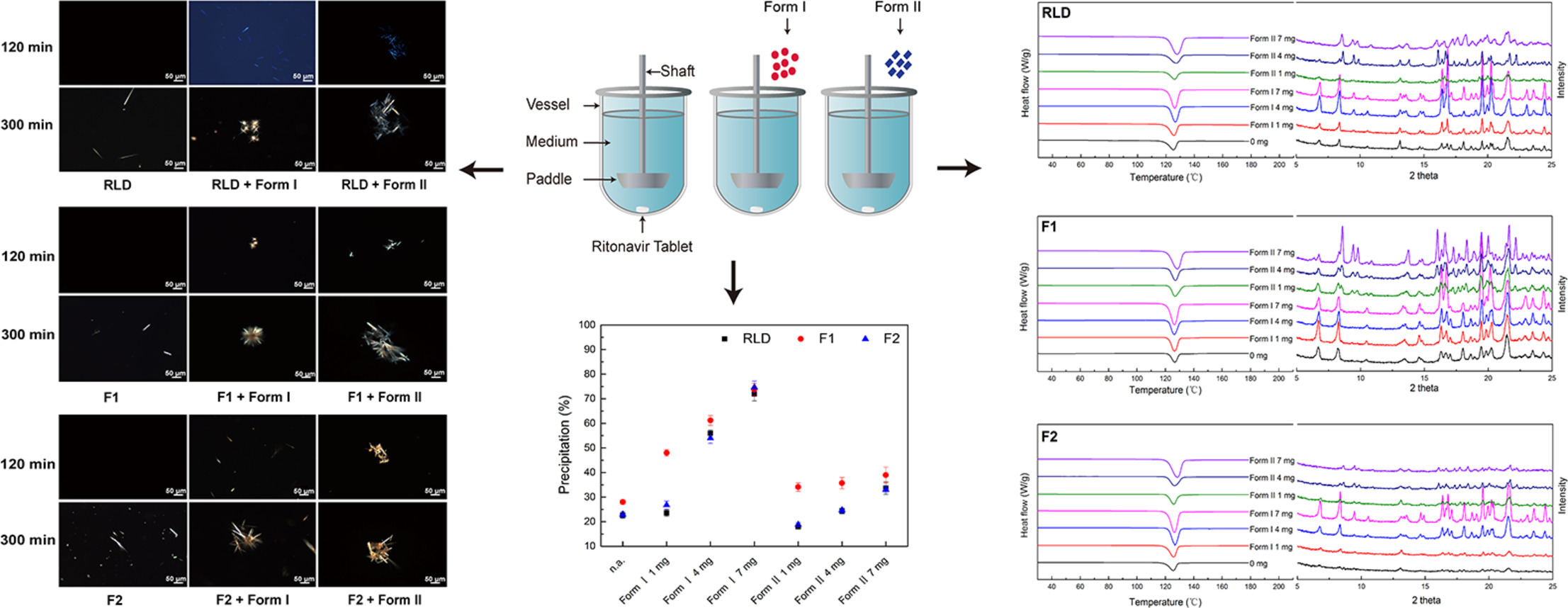
The metastable Form I was the preferred polymorph to precipitate without seed crystals.
The metastable Form I seed led to more precipitation compared to that of the stable Form II seed.
Form I precipitated from supersaturated solution were easily dispersed in the solution, which might in turn serve as seeds to facilitate the crystal growth.
The stable Form II crystals presented as aggregates tend to grow slowly.
The precipitation of Form I from supersaturated solution was somehow inhibited by Form II seeds.
The results showed that both Form I and Form II ritonavir tablets had similar dissolution profiles, which were also similar to the reference listed drug (RLD). However, it was observed that the presence of seed crystals, particularly the metastable Form I seed, led to more precipitation compared to the stable Form II seed in all formulations. The Form I crystals that precipitated from the supersaturated solution were easily dispersed in the solution and could serve as seeds to facilitate crystal growth. On the other hand, Form II crystals tended to grow more slowly and presented as aggregates. The addition of both Form I and Form II seeds could affect their precipitation behaviors, and the amount and form of the seeds had significant effects on the precipitation process of the RLD tablets, as are the tablets prepared with different polymorphs. In conclusion, the study highlights the importance of minimizing the contamination risk of seed crystals during the manufacturing process and selecting the appropriate polymorph for the production of ASDs.
Table 2. The list of ready-to-use excipients.
| Excipient Name | Type | Source |
|---|---|---|
| PVPVA | Kollidon® VA 64 | BASF Corporation, New Jersey, Germany |
| Sorbitan monolaurate | Span 20-LQ-(AP) | Croda Inc., East Yorkshire, UK |
| Colloidal Silicon Dioxide | AEROSIL® 200 Pharma | Evonik Degussa GmbH, Essen, Germany |
| Dibasic Calcium Phosphate Anhydrous | Fujicalin® SG | Fuji Chemical Industries Co., Ltd., Toyama, Japan |
| Sodium Stearyl Fumarate | Alubra® PG 100 | DuPont de Nemours, Inc., Delaware, USA |
Download the full article as PDF here Effect of different seed crystals on the supersaturation state of ritonavir tablets prepared by hot-melt extrusion
or read it here
Hengqian Wu, Zhengping Wang, Yanna Zhao, Yan Gao, Lili Wang, Heng Zhang, Rupeng Bu, Zhuang Ding, Jun Han,
Effect of different seed crystals on the supersaturation state of ritonavir tablets prepared by hot-melt extrusion, European Journal of Pharmaceutical Sciences, Volume 185, 2023, 106440, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2023.106440.
Read more on “Lubricants” here


