The Preparation and Evaluation of Cyanocobalamin Mucoadhesive Sublingual Tablets
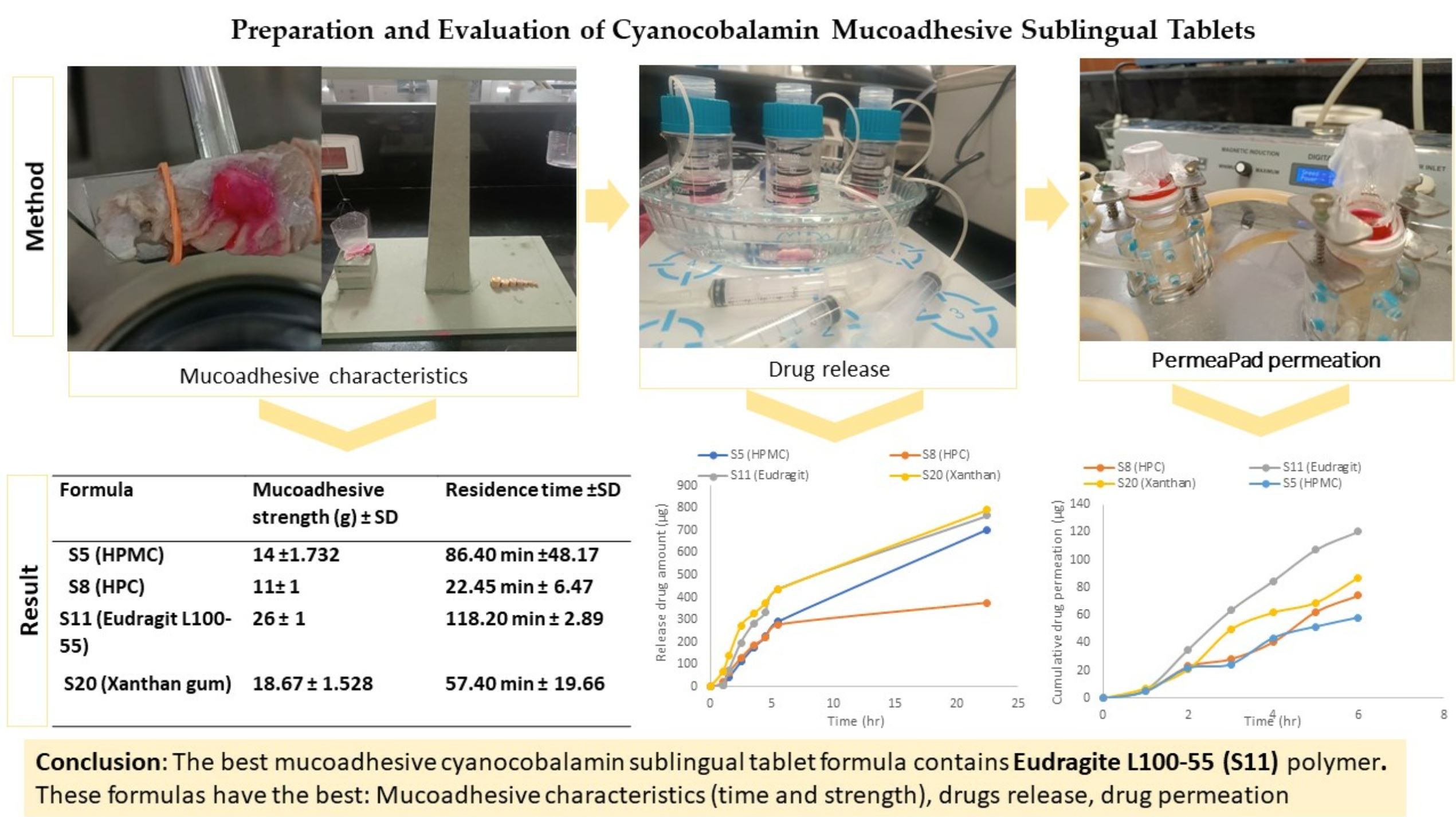
Cobalamin (vitamin B12), an essential vitamin with low oral bioavailability, plays a vital role in cellular functions. This research aimed to enhance the absorption of vitamin B12 using sublingual mucoadhesive tablets by increasing the residence time of the drug at the administration site. This research involved the preparation of different 50 mg placebo formulas using different methods. Formulas with disintegration times less than one minute and appropriate physical characteristics were incorporated into 1 mg of cyanocobalamin (S1–S20) using the direct compression method. The tablets obtained were evaluated ex vivo for residence time, and only those remaining for >15 min were included. The final formulas (S5, S8, S11, and S20) were evaluated in several ways, including pre- and post-compression, drug content, mucoadhesive strength, dissolution, and Permeapad® permeation test employed in the Franz diffusion cell.
After conducting the evaluation, formula S11 (Eudragit L100-55) emerged as the most favorable formulation. It exhibited a mucoadhesive residence time of 118.2 ± 2.89 min, required a detachment force of 26 ± 1 g, maintained a drug content of 99.124 ± 0.001699%, and achieved a 76.85% drug release over 22 h, fitting well with the Peppas–Sahlin kinetic model (R2: 0.9949). This suggests that the drug release process encompasses the Fickian and non-Fickian kinetic mechanisms. Furthermore, Eudragit L100-55 demonstrated the highest permeability, boasting a flux value of 6.387 ± 1.860 µg/h/cm2; over 6 h. These findings indicate that including this polymer in the formulation leads to an improved residence time, which positively impacts bioavailability.
Download the full article as PDF here The Preparation and Evaluation of Cyanocobalamin Mucoadhesive Sublingual Tablets
or read it here
Materials
Cyanocobalamin was donated by Planet Pharma, Eudragit (S100, L100, and L100-55) by Evonik Industries, and Mannitol by Pharmacare PLC. Hydroxy propylcellulose (M.W. 100,000) and hydroxy propyl methyl cellulose ((2% aq. Soln., 20 °C) 7500–14,000 mPa.s) were purchased from Alfa Aesar (Haverhill, MA, USA); ethyl cellulose, microcrystalline cellulose PH 101, polyplasdone, and magnesium stearate from Colorcon® (Milan, Italy); sublingual bovine mucosa from a local butcher (Palestine); PermeaPad® membrane from innoME GmbH (Espelkamp, Germany); dialysis tubing cellulose membrane and polyvinyl pyrrolidine (MW 40,000) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Xanthan gum, carbopol, sodium hydroxide pellets, sodium chloride, disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, absolute anhydrous ethanol 100%, acetonitrile, and hydrochloric acid 37% were obtained from Birzeit University laboratories (Ramallah, Palestine).
Ma’ali, A.; Naseef, H.; Qurt, M.; Abukhalil, A.D.; Rabba, A.K.; Sabri, I. The Preparation and Evaluation of Cyanocobalamin Mucoadhesive Sublingual Tablets. Pharmaceuticals 2023, 16, 1412. https://doi.org/10.3390/ph16101412
See our overview article on the CPHI 2023 Barcelona
with a focus on excipients:


