Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles
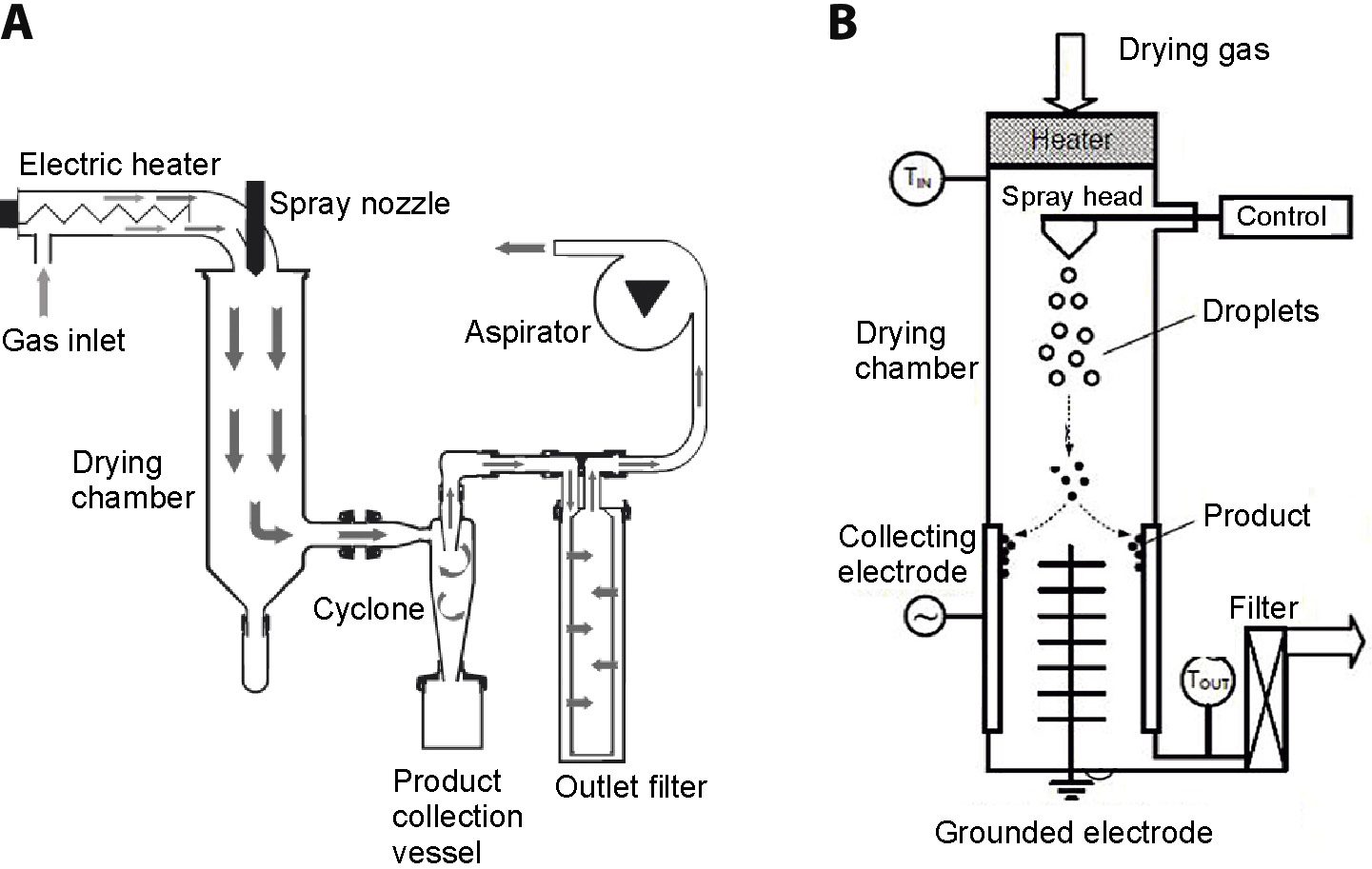
In this review, benefits and drawbacks of the process of spray drying and nano spray drying with regard to the manufacturing of polymeric particles for pharmaceutical applications are discussed. Spray drying has been used for many years in the food, chemical and pharmaceutical industries for converting liquids into solids, in order to form products of uniform appearance. The construction of spray dryer enables to atomize the liquid into small droplets, which ensures a large surface area for heat and mass transfer, and significantly shortens the processing.
Each droplet dries to an individual solid microparticle of characteristic features that can be tailored by optimizing formulation variables and critical process parameters. Since spray drying technology is easy to scale up and can be used for drying almost any drug in a solution or suspension, there are numerous examples of products in clinical use, in which this process has been successfully applied to improve drug stability, enhance bioavailability or control its release rate. In recent years, nano spray drying technology has been proposed as a method for lab-scale manufacturing of nanoparticles. Such an approach is of particular interest at early stages of drug development, when a small amount of new chemical entities is available. Here, the nebulization technique is used for feed atomization, while laminar gas flow in the drying chamber ensures gentle drying conditions. Moreover, electrostatic collectors have gradually replaced cyclone separators, ensuring high effectiveness in producing solid nanoparticles, even if a small volume of the sample is processed.
| Drug and route of administration | Carrier | Drug:carrier ratio (w/w) | Solvent | Process parameters | Yield [%] | Particle size [μm] | Particle morphology | Advantages |
|---|---|---|---|---|---|---|---|---|
| Carbamazepine34 for oral administration | Chitosan HPMC | 1:1 7:3 9:1 | for crude drug: ethanol 96% for samples loaded with HPMC: ethanol/water 2:3 (v/v) for samples loaded with chitosan: 0.5% acetic acid | inlet temperature: 120°C outlet temperature: 75°C spray flow rate: 0.25 L/h air flow rate: 700 NxL/h | ~30 | ~3 | Spherical microspheres | drug amorphization; faster drug release from chitosan-HPMC composite microparticles than those made of HPMC; sustained drug release possible |
| Andrographolide37 for oral administration | PVP | 1:2 1:3 1:4 | methanol | inlet temperature: 60°C outlet temperature: 45°C feed rate: 6-8 mL/min atomization air pressure: 2 kg/cm2 | 60-70 | 2.8-3.6 | spherical microparticles | micronization; drug amorphization; stabilizing effect of hydrogen bonds; 5-fold solubility increase |
| Felodipine38 for oral administration | PVPVA | 1:4 | acetone | inlet temperature: 72-184°C outlet temperature: 32-61°C feed rate: 110-188 g/min atomization air pressure: 2.11 kg/cm2 cyclone: 10.2 cm or 15.2 cm two-fluid nozzle or pressure swirl nozzle | 66-90 | 4-115 | intact, collapsed or fractured hollow spheres | drug amorphization; flowability of amorphous solid dispersions suitable for compaction; high mechanical resistance of tablets |
| Diltiazem39 for oral administration | Eudragit RS & Eudragit RL | 1:2 1:4 1:8 | DCM | inlet temperature: 70°C outlet temperature: 57-60°C feed rate: 2-5 mL/min spray-flow: 700 N x L/h 0.5 mm nozzle | N/A | 1-9 | smooth microspheres | narrow particle size distribution; drug amorphization; high drug load results in faster release rate |
| Caffeine or progesterone43 | PLA | for progesterone: 10:90 20:80 35:65 50:50 for caffeine: 25:75 40:60 60:40 75:25 | DCM | inlet temperature: 70°C outlet temperature: 40-45°C spray-flow: 600 N x L/h 0.5 mm nozzle | N/A | <5 | microparticles with progesterone: spherical; those loaded with caffeine: needle-shaped | drug microencapsulation; retarded drug release |
| Vancomycin7 for topical ocular delivery | PLGA | 1:2 1:3 1:4 | for drug: water for polymer: DCM | inlet temperature: 80-85°C outlet temperature: 68-70°C spray-rate: ~10 mL/min 0.7 mm nozzle | <55 | 10.96-11.75 | almost spherical particles with smooth surface, agglomerates visible | controlled ddrug release; enhanced pharmacokinetic parameters of drug from aqueous suspensions of microsheres shown in rabbit modes |
Read more
Strojewski D, Krupa A. Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles [published online as ahead of print on August 12, 2022]. Polim Med. 2022.
doi:10.17219/pim/152230
Read more Chitosan as a pharmaceutical excipient here:


