Leveraging a multivariate approach towards enhanced development of direct compression extended release tablets
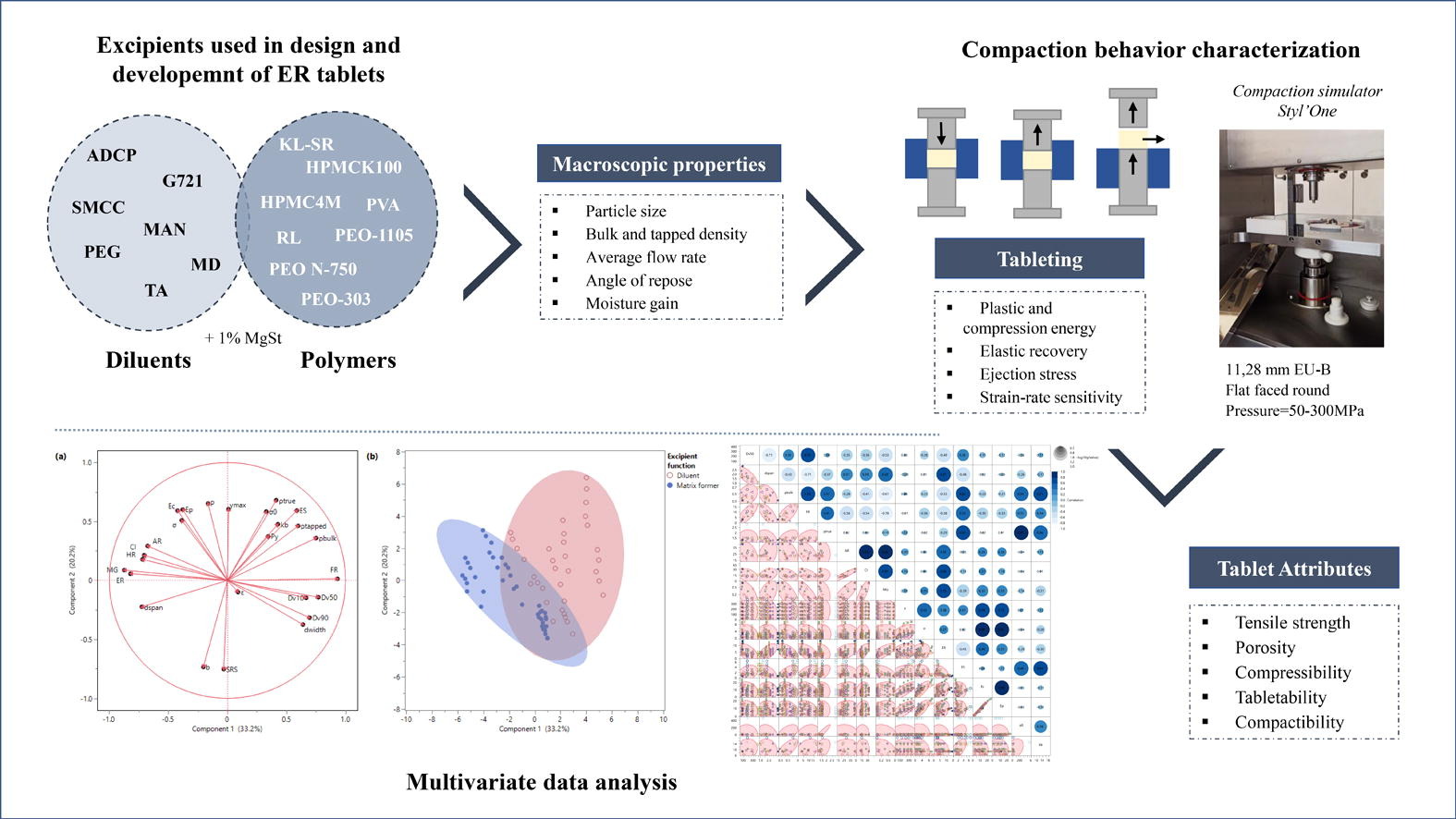
Extended release formulations play a crucial role in the pharmaceutical industry by maintaining steady plasma levels, reducing side effects, and improving therapeutic efficiency and compliance. One commonly used method to develop extended release formulations is direct compression, which offers several advantages, such as simplicity, time savings, and cost-effectiveness. However, successful direct compression-based extended release formulations require careful assessment and an understanding of the excipients’ attributes. The scope of this work is the characterization of the compaction behavior of some matrix-forming agents and diluents for the development of extended release tablets. Fifteen excipients commonly used in extended release formulations were evaluated for physical, compaction and tablet properties. Powder properties (e.g., particle size, flow properties, bulk density) were evaluated and linked to the tablet’s mechanical properties in a fully integrated approach, and data were analyzed by constructing a principal component analysis (PCA). Significant variability was observed among the various excipients. The present work successfully demonstrates the applicability of PCA as an effective tool for comparative analysis, pattern and clustering recognition and correlations between excipients and their properties, facilitating the development and manufacturing of direct compressible extended release formulations.
Download the full article as PDF here Leveraging a multivariate approach towards enhanced development of direct compression extended release tablets
or read it here
Materials
The materials (15) included in this study were purchased from or provided by BASF (Ludwigshafen, Germany), The Dow Chemical Company (Midland, MI, USA), Merck & Co. (Kenilworth, New Jersey, USA), JRS Pharma (Rosenberg, Baden-Wurttemberg, Germany), BENEO (Mannheim, Baden-Wurttemberg, Germany), Avesta Pharma Pvt. Ltd. (Mumbai, Maharashtra, India), Roquette (Lestrem, France), MEGGLE Pharma (Wasserburg am Inn, Germany) and UNDESA (Barcelona, Spain). Table 1 provides an overview of the raw materials. Chemical Abstracts Service (CAS) number and unique ingredient identifier (UNII) were used as identifiers linked to the substance’s molecular structure or descriptive information.
Table 1. Raw materials used in the study, their function and manufacturer.
| Abbreviation | Excipient name | Commercial name | Function | CAS N. | UNII | Manufacturer |
|---|---|---|---|---|---|---|
| KL-SR | Acetate polyvinyl 80% PVP 20% | Kollidon® SR | Matrix former | 9003-20-7 9003-39-8 | 32K497ZK2U | BASF |
| HPMCK4M | Hydroxypropyl methylcellulose | Methocel™ K4M Premium | Matrix former | 9004-65-3 | 3NXW29V3WO | DuPont |
| HPMCK100 | Hydroxypropyl methylcellulose | Methocel™ K100 Premium CR | Matrix former | 9004-65-3 | 3NXW29V3WO | DuPont |
| PEO-N750 | Poly(ethylene)oxide | POLYOX™ WSR N750 | Matrix former | 25322-68-3 | 4QIB4U4CQR | DuPont |
| PEO-1105 | Poly(ethylene)oxide | POLYOX™ WSR 1105 | Matrix former | 25322-68-3 | 16P9295IIL | DuPont |
| PEO-303 | Poly(ethylene)oxide | POLYOX™ WSR 303 | Matrix former | 25322-68-3 | G3MS6M810Y | DuPont |
| PVA | Polyvinyl alcohol | Parteck® SRP80 | Matrix former | 9002-89-5 | 532B59J990 | Merck & Co |
| RL | Lactose coprocessed | RetaLac® | Matrix former | 5989-81-1 9004-65-3 | EWQ57Q8I5X 39J80LT57T | MEGGLE |
| ADCP | Calcium hydrogen phosphate anhydrous | Emcompress® Anhydrous | Diluent | 7757-93-9 | L11K75P92J | JRS Pharma |
| G721 | Isomalt | galenIQ™ 721 | Diluent | 64519-82-0 | S870P55O2W | BENEO |
| TA-80 | Lactose Monohydrate | Tablettose® 80 | Diluent | 64044-51-5 | EWQ57Q8I5X | MEGGLE |
| MD-IT12 | Maltodextrin | Glucidex® IT 12 | Diluent | 9050-36-6 | 7CVR7L4A2D | Roquette |
| MAN-400DC | Mannitol | Pearlitol® 400 DC | Diluent | 69-65-8 | 3OWL53L36A | Roquette |
| PEG6000 | Poly(ethylene glycol) 6000 | Macrogol 6000 | Diluent | 25322-68-3 | 30IQX730WE | Avesta Pharma |
| SMCCHD90 | Silicified Microcrystalline Cellulose | PROSOLV® SMCC HD90 | Diluent | 9004-34-6 | OP1R32D61U | JRS Pharm |
| MgSt | Magnesium stearate | Kemilub EM-F-V | Lubricant | 557- 04-0 | 70097M6I30 | UNDESA |
A.S. Sousa, J. Serra, C. Estevens, R. Costa, A.J. Ribeiro, Leveraging a multivariate approach towards enhanced development of direct compression extended release tablets, International Journal of Pharmaceutics, 2023, 123432, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123432.
See the webinar:
“Fast Track development of Biphasic nano-dexamethasone Pellets using galenIQ™”, 12 October 2023:
Get more information & register here for free:


