Development of Lipid Polymer Hybrid Drug Delivery Systems Prepared by Hot-Melt Extrusion
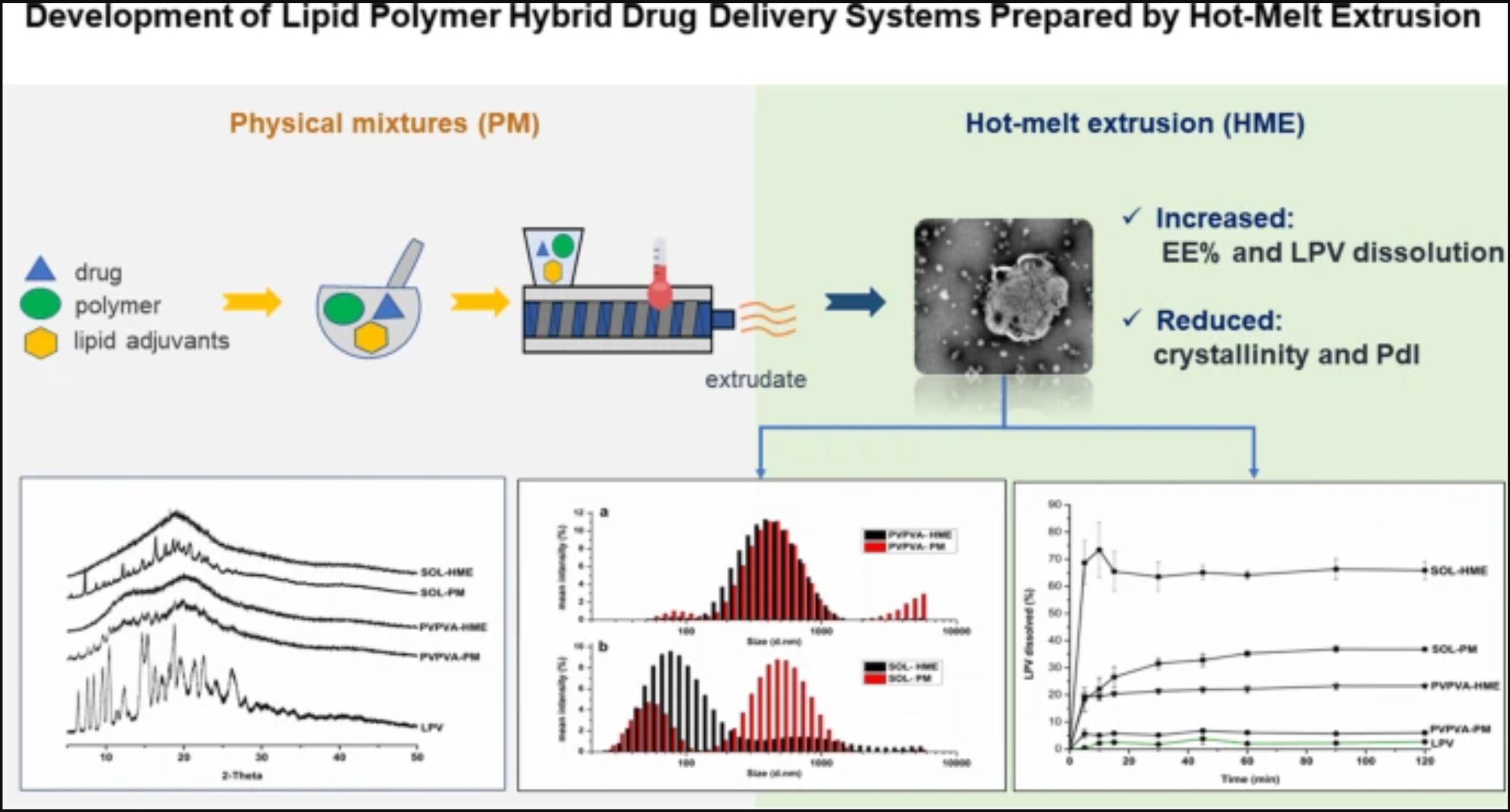
This study sought to develop polymer-lipid hybrid solid dispersions containing the poorly soluble drug lopinavir (LPV) by hot-melt extrusion (HME). Hence, the lipid and polymeric adjuvants were selected based on miscibility and compatibility studies. Film casting was used to assess the miscibility, whereas thermal, spectroscopic, and chromatographic analyses were employed to evaluate drug-excipient compatibility. Extrudates were obtained and characterized by physicochemical tests, including in vitro LPV dissolution. Preformulation studies led to select the most appropriate materials, i.e., the polymers PVPVA and Soluplus®, the plasticizers polyethylene glycol 400 and Kolliphor® HS15, phosphatidylcholine, and sodium taurodeoxycholate.
HME processing did not result in LPV degradation and significantly increased entrapment efficiency (93.8% ± 2.8 for Soluplus® extrudate against 19.8% ± 0.5 of the respective physical mixture). LPV dissolution was also increased from the extrudates compared to the corresponding physical mixtures (p < 0.05). The dissolution improvement was considerably greater for the Soluplus®-based formulation (24.3 and 2.8-fold higher than pure LPV and PVPVA-based extrudate after 120 min, respectively), which can be attributed to the more pronounced effects of HME processing on the average size and LPV solid-state properties in the Soluplus® extrudates. Transmission electron microscopy and chemical microanalysis suggested that the polymer-lipid interactions in Soluplus®-based formulation depended on thermal processing.
Read more here
Kasbaum, F.E., de Carvalho, D.M., de Jesus Rodrigues, L. et al. Development of Lipid Polymer Hybrid Drug Delivery Systems Prepared by Hot-Melt Extrusion. AAPS PharmSciTech 24, 156 (2023). https://doi.org/10.1208/s12249-023-02610-y
Read more articles on “Lipids” here: