Effect of process parameters in high shear granulation on characteristics of a novel co-processed mesoporous silica material
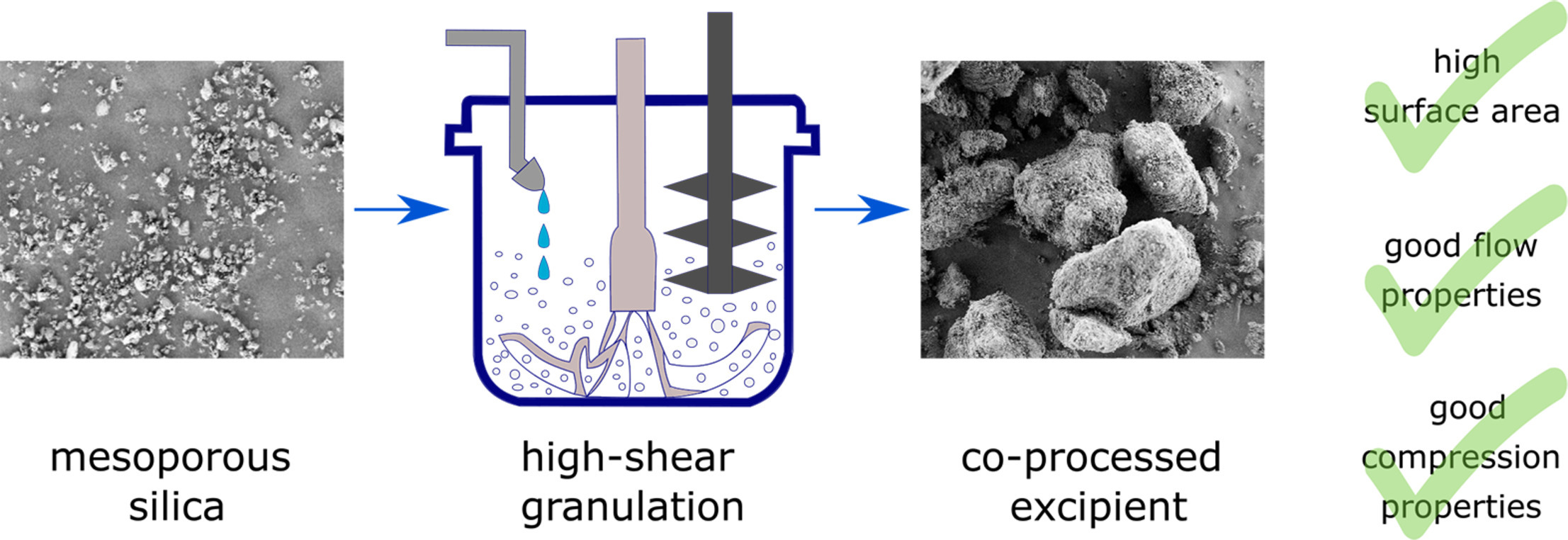
In this study, insights into the development and optimization of a co-processed excipient based on mesoporous silica are presented. The main advantage of such a material is that it is appropriate for direct tablet compression and has a sufficiently large specific surface area to be suitable for potential subsequent drug loading and formulation of (amorphous) solid dispersions. Our aim was to use a Design of Experiments approach to investigate which process parameters in high shear granulation affect the characteristics of such a co-processed material. The parameters included were the amount of binder (isomalt), the amount of water (granulation liquid), the water addition rate and the speed of the impeller.
Highlights
- Granulation of mesoporous silica and isomalt yields co-processed excipient
- Co-processed silica exhibits improved properties compared to the starting material
- Granulation process parameters greatly influence characteristics of the excipient
- Design of Experiment method gives a good insight into factors governing granulation
The responses evaluated and modelled were particle size and its distribution, specific surface area, bulk density, flowability, compressibility and compactibility. The models obtained showed good quality in terms of goodness of fit and predictive power. Active effects were identified for all responses, giving a thorough insight into factors affecting the material characteristics. Optimization experiments resulted in products with the desired characteristics (high specific surface area, large particle size, good flow and compression properties) and confirmed the validity of the generated models.
Table 1: Examples of co-processed excipients on the market
| Excipient name | Individual components and their content | Advantages, intention of use |
|---|---|---|
| Cellactose® | α-lactose monohydrate (75%) Powdered cellulose (25%) | Improved flowability and compactibility Direct compresison |
| Ludipress® | Lactose monohydrate (93%) Kolidon®30 (3.5%) Kollidon®CL (3.5%) | Improved flowability and tabletability, rapid disintgeration Direct compression |
| StarLac | Maize starch (15%) α-lactose monohydrate (85%) | Improved flowability, rapid disintegration Direct compression |
| Ludiflash® | D-Mannitol (84-92%) Kolldion®CL-SF (4-6%) Polyvinyl acetate (3.5-6%) Kollidon®30 (0.25-0.60%) Water (0.5-2.0%) | Rapid disintegration, pleasant taste Direct compression, fast disintegrating solid oral dosage forms |
| Pharmaburst® 500 | D-Mannitol (85%) Silicon dioxide (<10%) Sorbitol (<10%) Crospovidone (<5%) | Rapid disintegration, good compaction, good mouthfeel Orally disintegrating tablets |
Download the full article as PDF here: Effect of process parameters in high shear granulation on characteristics of a novel co-processed mesoporous silica material
or read it here
Material
Isomalt (GalenIQ 800) was kindly donated by Beneo (Germany). Mesoporous silica (Syloid 244 FP) was obtained from Grace Davison, Grace GmbH & Co. KG (Germany). Magnesium stearate was obtained from Merck KGaA (Germany). Water was purified by reverse osmosis.
Ana Baumgartner, Odon Planinšek, Effect of process parameters in high shear granulation on characteristics of a novel co-processed mesoporous silica material, European Journal of Pharmaceutical Sciences, 2023, 106528, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2023.106528.
Magnesium Stearate as pharmaceutical excipient – See our introduction video

