A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery
Fungal infections are an extremely serious health problem, particularly in patients with compromised immune systems. Most antifungal agents have low aqueous solubility, which may hamper their bioavailability. Their complexation with cyclodextrins (CDs) could increase the solubility of antifungals, facilitating their antifungal efficacy. Nanoparticulate systems are promising carriers for antifungal delivery due to their ability to overcome the drawbacks of conventional dosage forms. CD-based nanocarriers could form beneficial combinations of CDs and nanoparticulate platforms. These systems have synergistic or additive effects regarding improved drug loading, enhanced chemical stability, and enhanced drug permeation through membranes, thereby increasing the bioavailability of drugs.
Here, an application of CD in antifungal drug formulations is reviewed. CD-based nanocarriers, such as nanoparticles, liposomes, nanoemulsions, nanofibers, and in situ gels, enhancing antifungal activity in a controlled-release manner and possessing good toxicological profiles, are described. Additionally, the examples of current, updated CD-based nanocarriers loaded with antifungal drugs for delivery by various routes of administration are discussed and summarized.
Introduction
Fungal infections are prevalent causes of morbidity and mortality in humans. The prolonged use of antibiotics or immunosuppressive agents leads to the increased vulnerability of human beings and renders them prone to fungal infections. Infections caused by opportunistic fungi or other pathogens are classified as superficial, cutaneous, subcutaneous, mucosal, or systemic based on the severity [1,2]. The most common pathogens causing life-threatening fungal infections are Candida spp., Aspergillus spp., Fusarium spp., Cryptococcus spp., and Pneumocystis spp.They mostly relate to immunocompromised patients, such as immunodeficient patients receiving immunosuppressive therapy or patients with cancer undergoing chemotherapy [3,4].
Globally, almost one-fourth of the human population is affected by Candida. Candidiasis is the most common systemic and localized infection among hospitalized patients worldwide [5]. Among the Candida infections, oral candidiasis and vaginal candidiasis are the most common infections [6,7]. The occurrence rate of oral candidiasis has significantly risen with the use of immunosuppressive drugs and immunodeficiency diseases [8]. The reported morbidity and mortality rates of invasive fungal infections are 1.5 million patients yearly. According to a survey, 90% of deaths resulting from fungal infections are caused by Candida, Cryptococcus, Aspergillus, and Pneumocystis [9]. In certain geographic regions, Blastomyces, Coccidioides, Histoplasma, Talaromyces, Paracoccidioides, and Sporotrichosis are the fungal pathogens causing life-threatening endemic mycoses [1,10].
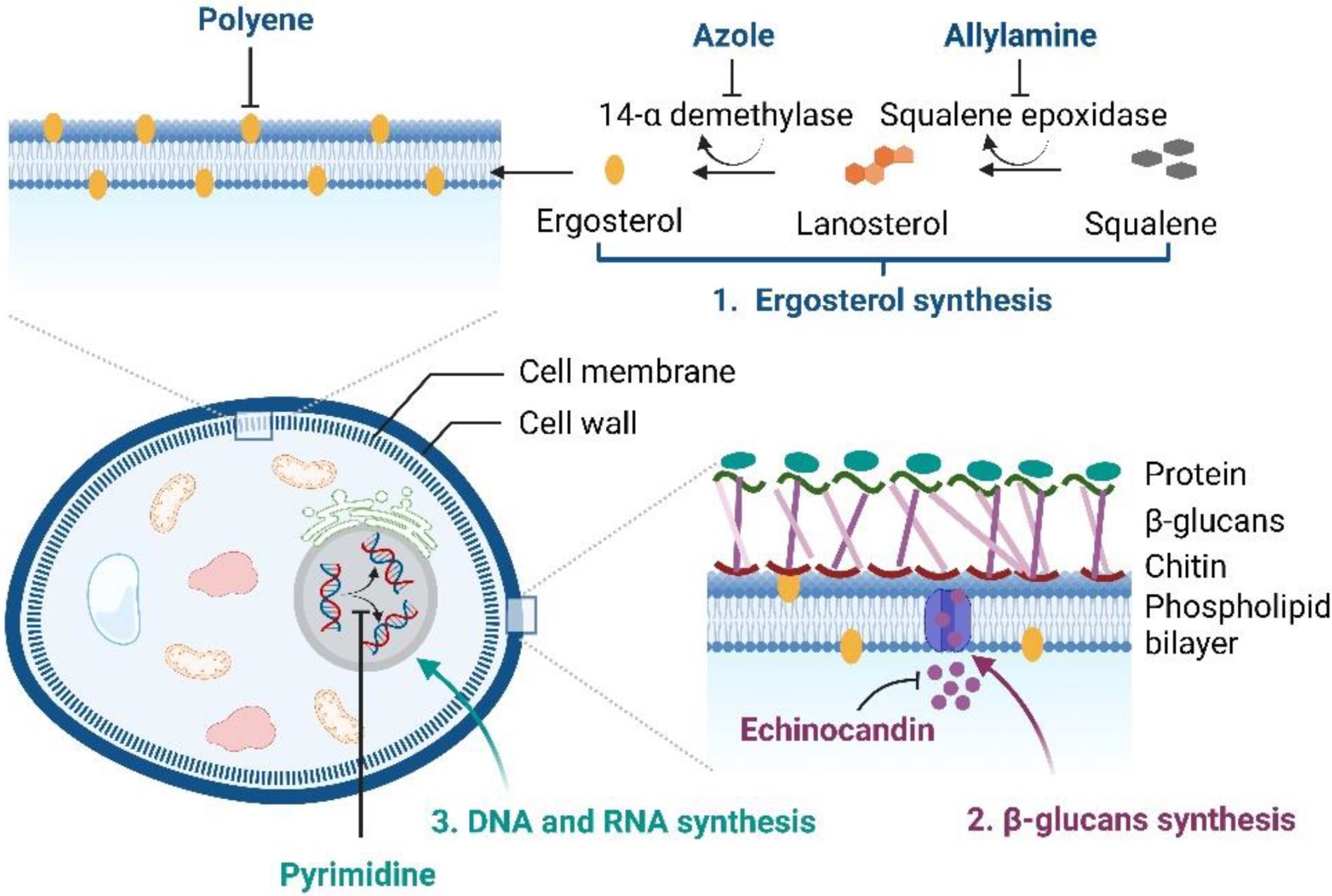
At present, clinically-applied antifungal drugs comprise five categories, i.e., azoles, polyenes, echinocandins, allylamines, and pyrimidine analogs, which are used to treat superficial and systemic fungal infections [11,12]. The antifungal activity of azoles (imidazole or triazoles antifungals) is achieved by inhibiting the sterol 14α-demethylase, an essential enzyme required for sterol biosynthesis, converting lanosterol to ergosterol, thereby destroying the stability and fluidity of fungal cell membranes. Amphotericin B, nystatin, and natamycin, which are polyene antifungal agents, bind to the fungal cell membrane and lead to pore formation and cell death.
The echinocandins (caspofungin, micafungin, and anidulafungin) are semisynthetic compounds that block fungal cell wall synthesis, whereas allylamine antifungals, i.e., terbinafine and naftifine, inhibit fungal growth by obstructing squalene epoxidase, an enzyme required for ergosterol biosynthesis [2,13]. Another class of antifungal drugs, i.e., pyrimidine analogs (5-fluorocytosine and 5-fluorouracil), exhibit antifungal activity by blocking DNA and RNA protein synthesis in fungal cell membranes [14]. The brief mechanisms of the actions of antifungals are illustrated in Figure 1.
Almost all of the above antifungals possess limitations due to their physicochemical properties, especially low aqueous solubility and permeability, leading to poor bioavailability and clinical efficacy [15,16]. Another critical factor is that, to achieve effective therapeutic action, a sufficient drug concentration at the site of infection is necessary. To address these issues, researchers have developed formulations or nanocarrier systems incorporating cyclodextrins (CDs). CDs are well-known cyclic oligosaccharides that can form water-soluble inclusion complexes with lipophilic compounds [17].
The solubility enhancement of poorly water-soluble drugs and the permeability enhancement of drugs through biological membranes are attained via the formation of drug/CD inclusion complexes [18]. Thus, CDs could facilitate the drug efficacy and bioavailability of many antifungal drugs [19,20,21]. In this present review, we focused on the updated information of currently available antifungal drug-loaded CD-based nanocarriers or formulations that are applied in different drug delivery systems to treat various fungal diseases effectively.
Table 2. Summarized data of the stability constant (K1:1 and K1:2) and complexation efficiency (CE) values of antifungal/CD complexes.
| Drug | CD a | Medium | pH | Type | K1:1 (M−1) | K1:2 (M−1) | CE | Refs. |
| Miconazole | αCD | Water (25 °C) | – | AL | 333 | – | – | [32] |
| Water (25 °C) | – | AL | 436 | – | 0.21 | [33] | ||
| βCD | Water (25 °C) | – | AL | 293 | – | – | [32] | |
| Water (25 °C) | – | BS | 596 b | – | 0.29 | [33] | ||
| Water (37 °C) | – | AL | 6065 | – | 0.902 | [34] | ||
| Phosphate buffer | pH 6 | AL | 97 | – | – | [35] | ||
| pH 7 | AL | 82 | – | – | ||||
| pH 8 | AL | 65 | – | – | ||||
| pH 9 | AL | 39 | – | – | ||||
| γCD | Water (25 °C) | – | AL | 695 | – | – | [32] | |
| Water (25 °C) | – | AN | 488 | – | 0.24 | [33] | ||
| HPβCD | Water (25 °C) | – | AL | 363 | – | – | [32] | |
| Water (37 °C) | – | AL | 1017 | – | 0.361 | [34] | ||
| HPγCD | Water (25 °C) | – | AL | 305 | – | – | [32] | |
| HEβCD | Water (25 °C) | – | AL | 312 | – | – | [32] | |
| Econazole | αCD | Water (22–23 °C) | pH 3 | AL | 354.5 | – | 0.371 | [36] |
| pH 5 | AL | 2597.5 | – | 0.293 | ||||
| pH 7.5 | AP | 870.2 | 15.0 | 0.041 | ||||
| Water (25 °C) | – | AL | 505.54 | – | 0.075 | [37] | ||
| Phosphate buffer saline (25 °C) | pH 7.4 | AL | Jun 28 | – | 0.036 | |||
| γCD | Water (22–23 °C) | pH 3 | BS | 246.7 b | – | 0.258 | [36] | |
| pH 5 | BS | 1032.8 b | – | 0.117 | ||||
| pH 7.5 | – c | – c | – c | – c | ||||
| HPβCD | Water (25 °C) | – | AL | 52.21 | – | 0.077 | [37] | |
| Phosphate buffer saline (25 °C) | pH 7.4 | AL | 86.34 | – | 0.051 | |||
| HPγCD | Water (25 °C) | – | AL | 54.11 | – | 0.080 | [37] | |
| Phosphate buffer saline (25 °C) | pH 7.4 | AL | 40.35 | – | 0.025 | |||
| Ketoconazole | βCD | Water (25 °C) | – | AL | 1859 | – | – | [38] |
| Water (37 °C) | – | AL | 4966 | – | – | [39] | ||
| SBEβCD | Water | – | AL | 843 | – | 0.399 | [40] | |
| Itraconazole | βCD | Phosphate buffer (25 °C) | pH 7.4 | AL | 885 | – | – | [41] |
| HPβCD | Water (25 °C) | pH 2 | AP | 5280 | 38 | – | [42] | |
| pH 4 | AP | 15 | 2504 | – | ||||
| pH 7 | AP | 1926 | 1 | – | ||||
| Voriconazole | αCD | Water (25 °C) | – | AL | 55.14 | – | 0.07 | [43] |
| HPβCD | Water (25 °C) | – | AL | 224.27 | – | 0.29 | [43] | |
| Water (37 °C) | – | AL | 320 | – | – | [44] | ||
| SBEβCD | Water (25 °C) | – | AL | 324.98 | – | 0.741 | [45] | |
| HPγCD | Water (25 °C) | – | AL | 242.65 | – | 0.32 | [43] | |
| Posaconazole | βCD | Water (15 °C) | – | AP | 296.66 | 935.42 | – | [46] |
| Water (25 °C) | – | AP | 300.28 | 983.19 | – | |||
| Water (37 °C) | – | AP | 307.12 | 1025.44 | – | |||
| HPβCD | Water (15 °C) | – | AP | 441.23 | 1289.18 | – | [47] | |
| Water (25 °C) | – | AP | 494.67 | 1337.24 | – | |||
| Water (37 °C) | – | AP | 431.36 | 1385.47 | – | |||
| DMβCD | Water (15 °C) | – | AP | 393.25 | 1269.53 | – | [46] | |
| Water (25 °C) | – | AP | 398.13 | 1296.27 | – | |||
| Water (37 °C) | – | AP | 405.86 | 1340.29 | – | |||
| Amphotericin B | αCD | Water (25 °C) | – | AL | 146 | – | 0.002 | [48] |
| βCD | Water (25 °C) | – | AL | 72.1 | – | 0.001 | [48] | |
| γCD | Water (25 °C) | – | AP | 4972.3 | 14. Jan | 0.069 | [48] | |
| Water (25 °C) | – | AP | 462 | 42 | – | [49] | ||
| Water (25 °C) | – | AL | 1129 | – | 0.016 | [50] | ||
| HPγCD | Water (25 °C) | – | AP | 2851.7 | 17.0 | 0.039 | [48] | |
| Nystatin | αCD | Water (25 °C) | – | BS | – c | – c | – c | [51] |
| βCD | Water (25 °C) | – | AL | 0.375 | – | – | [51] | |
| γCD | Water (25 °C) | – | AL | 0.539 | – | – | [51] | |
| Natamycin | βCD | Water (25 °C) | – | AL | 1010 | – | – | [52] |
| γCD | Water (25 °C) | – | AN | – c | – c | – c | [52] | |
| Water (25 °C) | – | AL | 667 | – | – | [53] | ||
| HPβCD | Water (25 °C) | – | AN | – c | – c | – c | [52] | |
| Flucytosine | βCD | Water (22 °C) | – | AL | 70 | – | – | [54] |
| HPβCD | Water (22 °C) | – | AL | 297 | – | – | [54] | |
| Terbinafine | αCD | 0.05 M Disodium hydrogen phosphate and 1M of Sodium hydroxide (25 °C) | pH 12 | AP | 02. Aug | 01. Jan | – | [55] |
| βCD | 0.05 M Disodium hydrogen phosphate and 1M of Sodium hydroxide (25 °C) | pH 12 | BS | 25 | – | – | [55] | |
| γCD | 0.05 M Disodium hydrogen phosphate and 1M of Dodium hydroxide (25 °C) | pH 12 | AL | 0.66 | – | – | [55] | |
| HPβCD | 0.05 M Disodium hydrogen phosphate and 1M of Dodium hydroxide (25 °C) | pH 12 | AL | 23 | – | – | [55] | |
| MβCD | 0.05 M Disodium hydrogen phosphate and 1M of Dodium hydroxide (25 °C) | pH 12 | AP | 46 | – | – | [55] |
Download the full article as PDF here A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery
or read it here
Soe, H.M.S.H.; Maw, P.D.; Loftsson, T.; Jansook, P. A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery. Pharmaceuticals 2022, 15, 1447. https://doi.org/10.3390/ph15121447

