Polymer selection for amorphous solid dispersion of a new drug candidate by investigation of drug polymer molecular interactions
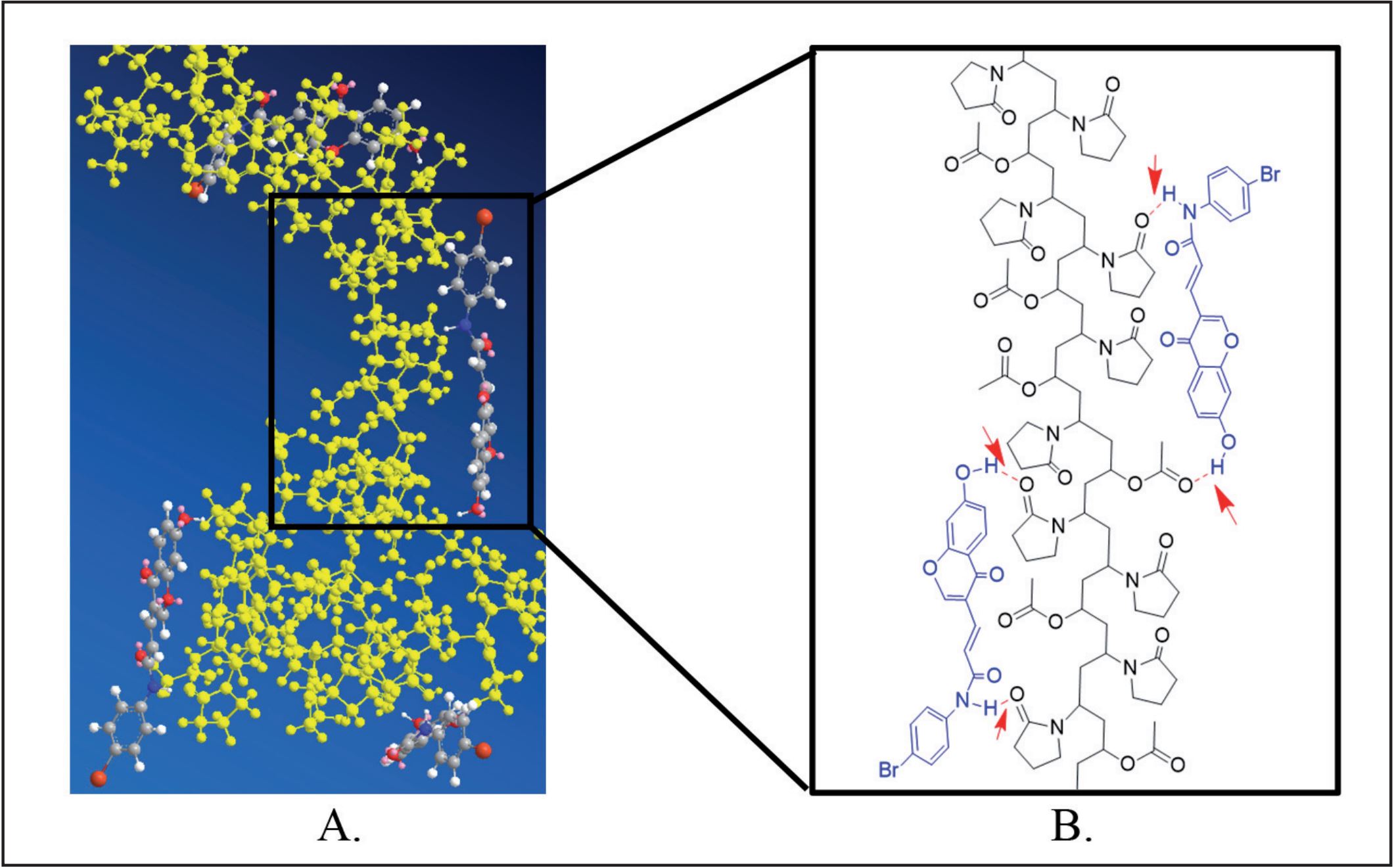
The antitumor drug candidate X-05 is being developed as an innovative anti-lung cancer drug candidate due to its excellent antitumour activity. A Caco-2 cell permeability study and solubility study confirmed that X-05 belonged to BCS class II or IV compounds. Therefore, the main challenge is to develop appropriate preparations for preclinical studies and further clinical phase research. By evaluating the preliminary results of kinetic solubility in biorelevant media and the structural analysis of X-05 and polymers, three polymers PVP K30, PVP VA 64 and HPMCAS, which may have intermolecular interactions with X-05, were chosen to select the optimal carrier for X-05 to prepare amorphous solid dispersions (ASDs).
ASD X-05-PVP VA 64 was selected as the optimal polymer by evaluating its kinetic solubility in biorelevant media and solid stability. The physical and chemical properties of ASD X-05-PVP VA 64 remain stable when the drug loading is as high as 50%. The drug-polymer interactions of ASD X-05-PVP VA 64 were studied by ultraviolet spectrophotometry, nuclear magnetic resonance spectrometry, infrared and Raman spectrophotometry, and the results indicated that the intermolecular hydrogen bond interaction between the drug and polymer was the foundation of the solubilization and stabilization of X-05 in PVP VA 64
Download the full article as PDF here Polymer selection for amorphous solid dispersion of a new drug candidate by investigation of drug polymer molecular interactions
Investigation
X-05 ASDs were prepared by spray drying with Kollidon® 30 (PVP K30), Kollidon® VA 64 (PVP VA 64) and hypromellose acetate succinate (AquaSolve MG) (HPMCAS). This section is divided into four parts. First, we discuss the characterization of X-05 ASDs in different polymers with polarized light microscopy (PLM), X-ray powder diffraction (XRPD) and thermal analysis; next, we discuss the kinetic solubility of X-05 ASDs in Fasted State Simulated Intestinal Fluid (FaSSIF), Fed State Simulated Intestinal Fluid (FeSSIF), and Simulated Gastric Fluid (SGF); then, we discuss the solid-state stability of X-05 ASDs in high temperature/humidity for as long as four weeks; last, we discuss the interactions between the molecules of X-05 and PVP VA 64 to understand the stabilization of X-05 in PVP VA 64 with high drug loading.
BIN HU, ZHILIANG LV2, GUILIANG CHEN, JIANZHONG LU, Polymer selection for amorphous solid dispersion of a new drug candidate by investigation of drug polymer molecular interactions, Received September 30, 2022, accepted August 11, 2023, Pharmazie 78: 185-195 (2023), doi: 10.1691/ph.2023.2061
Read articles on our category “Stabilizer” here:
- Microemulsions: An Encapsulation Strategy to Increase the Thermal Stability of D-limonene
- Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery
- Nanosuspensions as carriers of active ingredients: Chemical composition, development methods, and their biological activities

