Self-microemulsifying drug delivery system as carrier for the oral delivery of glimepiride: Formulation development, optimization, in-vitro characterization, stability assessment, ex-vivo permeation, and in-vivo antidiabetic activity in albino mice
Abstract
Aim: The research aimed to design the glimepiride self-micro emulsifying drug delivery system (SMEDDS) for increased oral bioavailability in albino mice by assessing hypoglycemic efficacy.
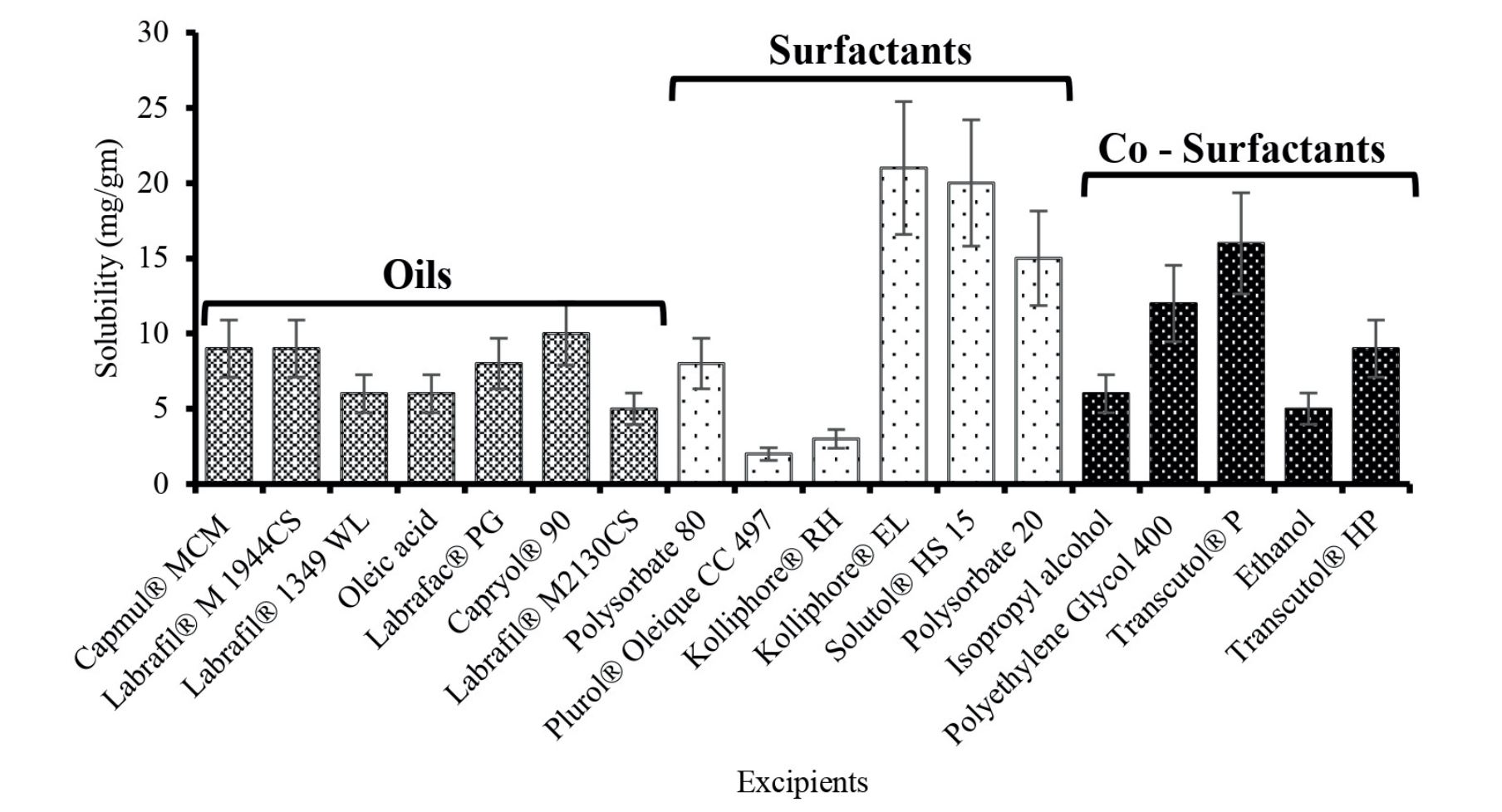
Materials and Methods: The optimized liquid SMEDDS (L-SMEDDS) prepared by emulsification of Capryol® 90 (oil), Kolliphor® EL (surfactant), and Transcutol® P (co-surfactant) were screened based on their solubility in glimepiride. Adsorption onto Aerosil® 200 Pharma produced solid SMEDDS (S-SMEDDS), and further direct compression was used to manufacture the tablets. The formulations were subjected to droplet size, polydispersity index (PdI), time of emulsification, in-vitro drug release, crystallinity nature, surface morphology, thermal behavior, ex vivo permeability, and in-vivo hypoglycemic activity in albino mice.
Results: The SMEDDS emulsified in less than 30 sec and had an average droplet size of 22.3 nm with a PdI of 0.296. The solid-state analysis revealed that glimepiride was in a molecular dispersion or amorphous form. In-vitro release experiments demonstrated that glimepiride released more efficiently than plain glimepiride. Both L-SMEDDS and S SMEDDS did not have any significant differences in terms of release. The ex-vivo and in-vivo studies revealed that SMEDDS possess improved permeability and hypoglycemic activity than plain glimepiride.
Conclusion: The study proved the combination of lipid-based nanosystems with oral dosage forms improved the oral bioavailability of glimepiride SMEDDS.
Download the full article here: Self-microemulsifying drug delivery system as carrier for the oral delivery of glimepiride
or read it here
Materials
Glimepiride was graciously provided as a complimentary sample by Alkem Laboratories Limited, India. Capmul® MCM was received as a generous gift from Abitec Corporation, India. Additionally, Labrafil® M 1944CS, Labrafil® 1349 WL, Labrafac® PG, Capryol® 90, Labrafil® M2130CS, Plurol® Oleique CC 497, Transcutol® P, and Transcutol® HP were generously provided by Gattefosse India Private Limited, India. The sources of Kolliphor® RH, Kolliphor® EL, Solutol® HS 15, and Polyplasdone® were BASF India Limited, India. Polysorbate 80, Polysorbate 20, and Polyethylene Glycol 400 were procured from SD Fine Chemicals Limited, India. SuperTab® 21 AN was kindly supplied by DFE Pharma India Limited, India. Avicel® PH 102 was received as a thoughtful gift from FMC Biopolymer India Private Limited, India. Starch 1500® was obtained as a sample from Colorcon Asia Private Limited, India. Aerosil® 200 Pharma was sourced from Evonik Industries Private Limited, India. Talc Luzenac was procured from Imerys Ceramics (India) Private Limited, India. Hyqual® magnesium stearate was generously provided by Mallinckrodt Pharmaceuticals, USA. Oleic acid, Isopropyl Alcohol, Methanol, Ethanol, and glucose powder were purchased from Loba Chemie Private Limited, India. Saline was obtained from Baxter Pharmaceuticals India Private Limited, India. Purified water, 0.1 hydrochloric acid (HCl) phosphate buffers, and tyrode solutions were prepared in-house.
Bhairy S, Mane S, Padhye S. Self-microemulsifying drug delivery system as carrier for the oral delivery of glimepiride: Formulation development, optimization, in-vitro characterization, stability assessment, ex-vivo permeation, and in-vivo antidiabetic activity in albino mice. Atlantic J Med Sci Res. 2024;4(1):9-18. DOI: 10.5455/atjmed.2023.09.051
Read also more on DC (Direct Compression) Excipients here:


