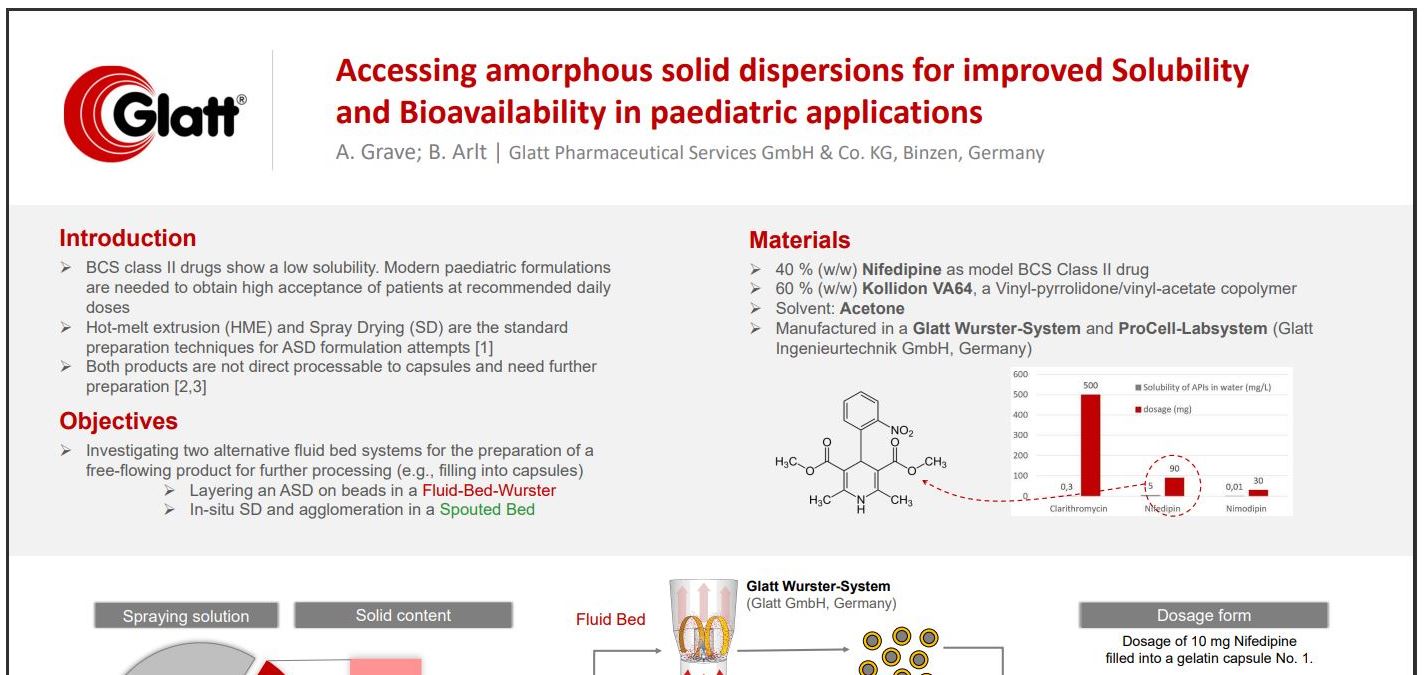
Accessing amorphous solid dispersions for improved Solubility and Bioavailability in paediatric applications
This article shows the content of a poster which was presented at the 𝗘𝗨𝗣𝗳𝗜 𝗰𝗼𝗻𝗳𝗲𝗿𝗲𝗻𝗰𝗲 𝗶𝗻 𝗚𝗹𝗮𝘀𝗴𝗼𝘄 2023
Introduction
- BCS class II drugs show a low solubility. Modern paediatric formulations are needed to obtain high acceptance of patients at recommended daily doses
- Hot-melt extrusion (HME) and Spray Drying (SD) are the standard preparation techniques for ASD formulation attempts [1]
- Both products are not direct processable to capsules and need further preparation [2,3]
Objectives
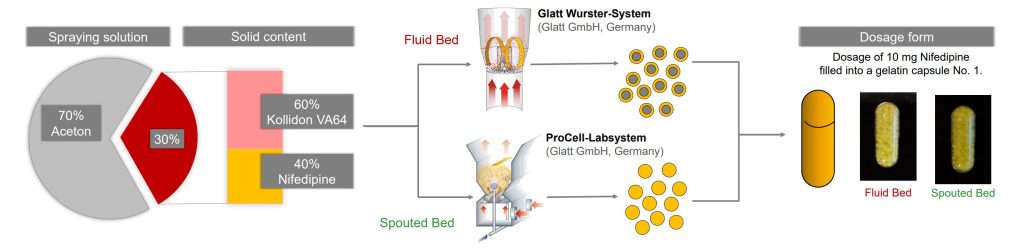
- Investigating two alternative fluid bed systems for the preparation of a free-flowing product for further processing (e.g., filling into capsules)
- Layering an ASD on beads in a Fluid-Bed-Wurster
- In-situ SD and agglomeration in a Spouted Bed
Materials
- 40 % (w/w) Nifedipine as model BCS Class II drug
- 60 % (w/w) Kollidon VA64, a Vinyl-pyrrolidone/vinyl-acetate copolymer
- Solvent: Acetone
- Manufactured in a Glatt Wurster-System and ProCell-Labsystem (Glatt Ingenieurtechnik GmbH, Germany)
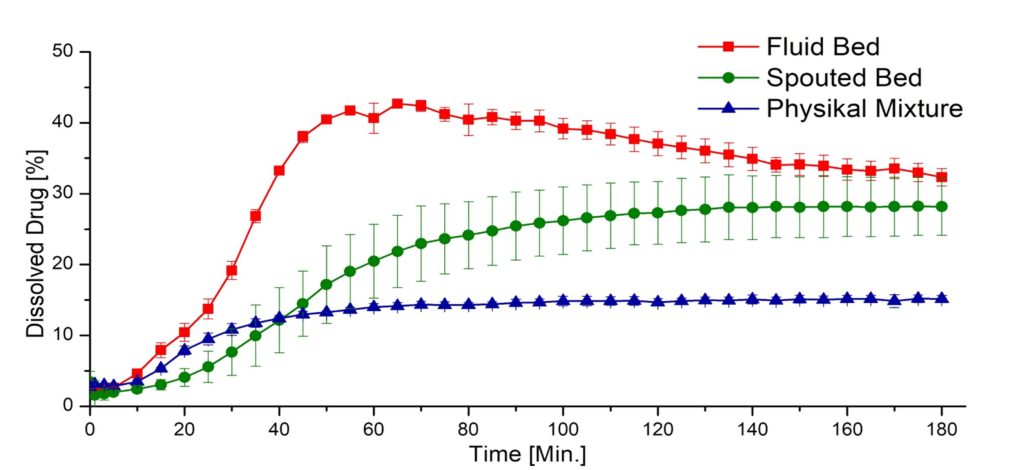
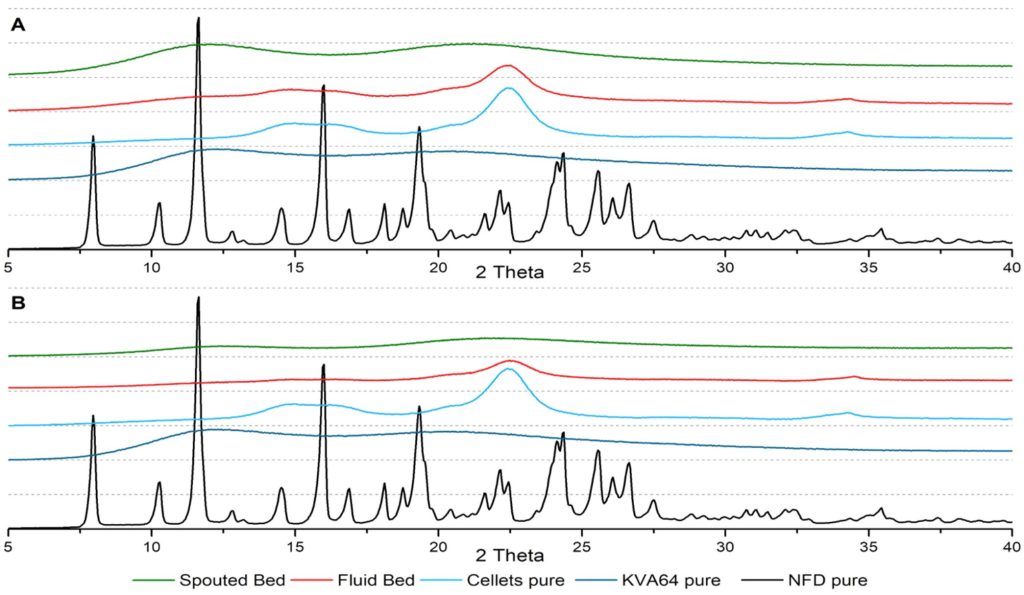
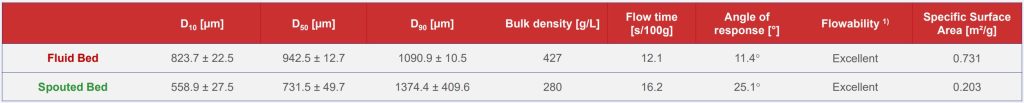
Characterization of the ASDs
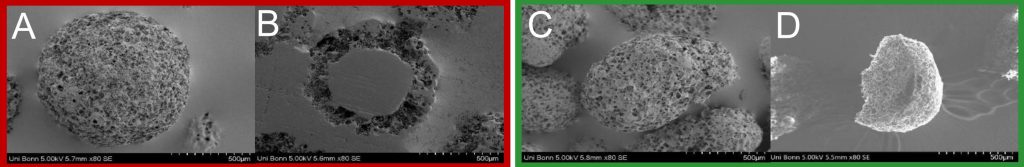
SEM Images
Conclusion
- Both techniques proved to be promising tools for thedevelopment and manufacturing of ASDs
• Stable over a long time (up to 2 yrs.)
• Good flow properties for further processing to e.g. capsules - Good dissolution performance
- Up-scaling to pilot or production scale possible
- Suitable for batch and continuous process design
See the full poster on “Accessing amorphous solid dispersions for improved Solubility and Bioavailability in paediatric applications” here
(click the picture to enlarge the poster)
Source: A. Grave; B. Arlt | Glatt Pharmaceutical Services GmbH & Co. KG, Binzen, Germany,
Poster “Accessing amorphous solid dispersions for improved Solubility and Bioavailability in paediatric applications”