Ternary solid dispersions of lacidipine: Enhancing dissolution and supersaturation maintenance through strategic formulation optimization
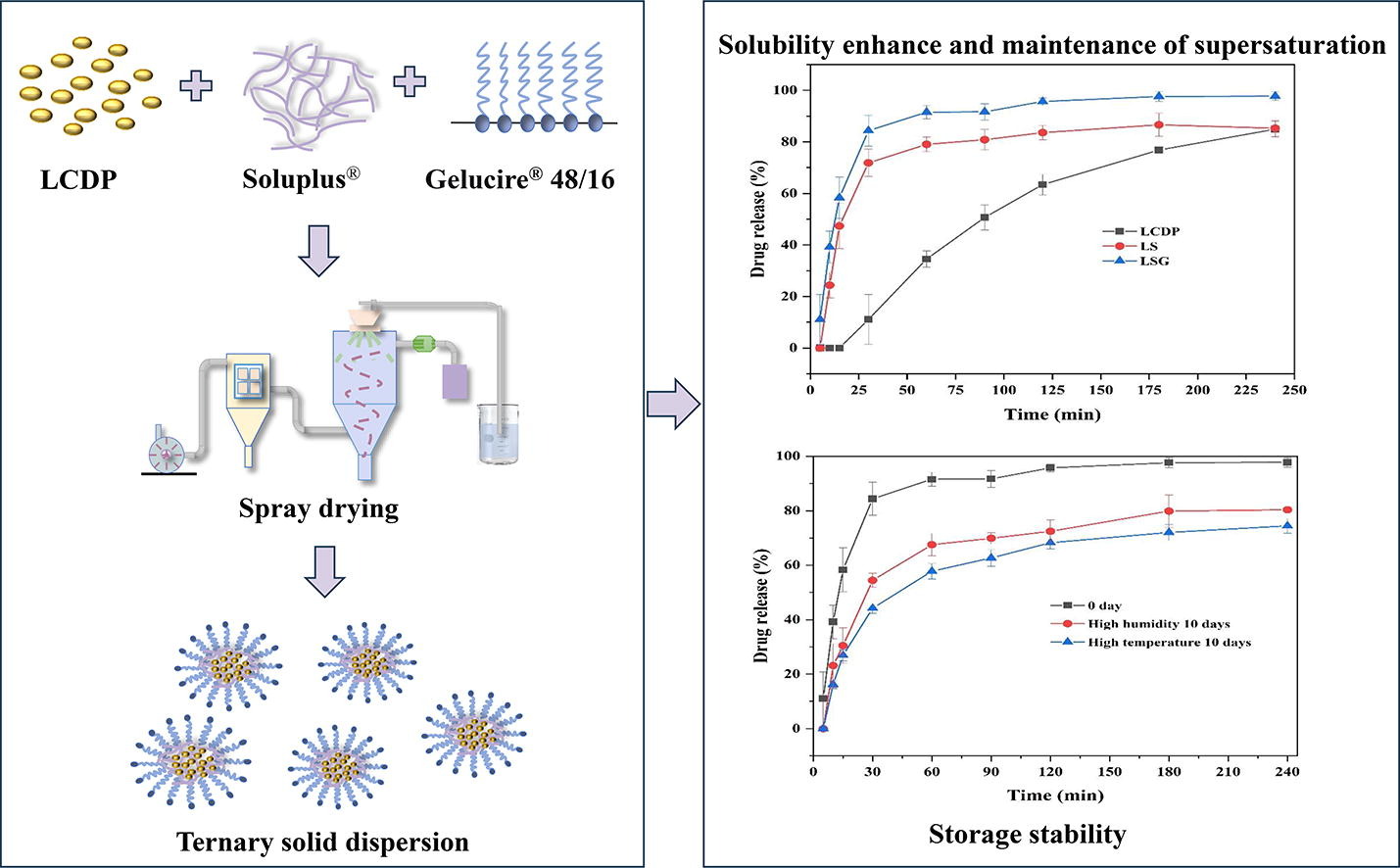
The study aimed to address the challenges related to insufficient dissolution and maintenance of supersaturation in binary solid dispersions. Lacidipine, categorized as a BCS class II drug, was employed as the model drug. A systematic screening of excipients was conducted to determine the most effective carriers for the formulations of the ternary solid dispersions, utilizing the solvent transfer method and equilibrium solubility measurements. Both binary and ternary solid dispersions were prepared via spray drying, and comprehensive physicochemical characterization confirmed the successful preparation of amorphous solid dispersions.
In vitro dissolution tests, the ternary solid dispersion exhibited marked superiority over the binary solid dispersion in dissolution and maintenance of supersaturation. Furthermore, an exploration into the factors influencing the stability of ternary solid dispersions revealed their robust resistance under light-protected, room-temperature, and desiccated conditions. The formation of intermolecular hydrogen bonding within the molecules of the ternary solid dispersions significantly enhanced drug solubility and system stability. Strategic formulation optimization, coupled with judicious selection of suitable carrier types and ratios, may serve as a promising approach for designing supersaturated drug delivery systems.
Read more here
Materials
LCDP was obtained from Sarn Chemical Technology Co., Ltd (Shanghai, China). PVP K-17 was obtained from Shanghai yuanye Bio-Technology Co., Ltd. PVP K-30 was obtained from ‘Beijing Wokai Biotechnology Co., Ltd. Kollidon®VA64 and Soluplus® was obtained from BASF China Limted. Poloxamer was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Tween 20 and dimethylformamide was purchased from Sinopharm Chemical Reagent Co., Ltd. Gelucire® 44/14 and Gelucire® 48/16 were provided by Gattefosse.
Jian Shen, Anna Hu, Yuxin Yang, Ting Nie, Siqi Huang, Zeneng Cheng, Wenjie Liu, Ternary solid dispersions of lacidipine: Enhancing dissolution and supersaturation maintenance through strategic formulation optimization,
International Journal of Pharmaceutics, 2024, 123989, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123989.
See our next webinar and register here:


