The clinical and translational prospects of microneedle devices, with a focus on insulin therapy for diabetes mellitus as a case study
Abstract
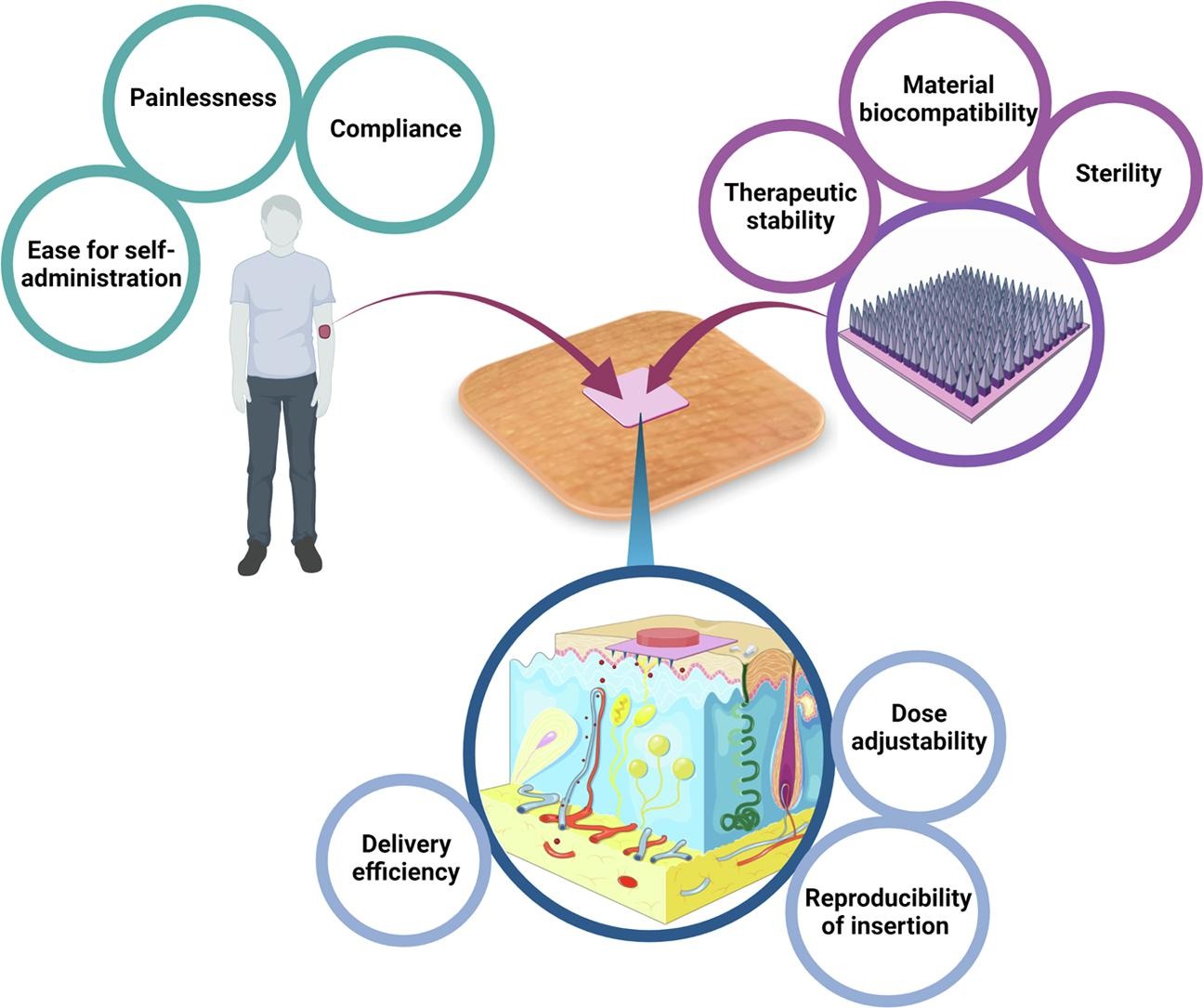
Microneedles have the clinical advantage of being able to deliver complex drugs across the skin in a convenient and comfortable manner yet haven’t successfully transitioned to medical practice. Diabetes mellitus is a complicated disease, which is commonly treated with multiple daily insulin injections, contributing to poor treatment adherence. Firstly, this review determines the clinical prospect of microneedles, alongside considerations that ought to be addressed before microneedle technology can be translated from bench to bedside. Thereafter, we use diabetes as a case study to consider how microneedle-based-technology may be successfully harnessed. Here, publications referring to insulin microneedles were evaluated to understand whether insertion efficiency, angle of insertion, successful dose delivery, dose adjustability, material biocompatibility and therapeutic stability are being addressed in early stage research. Moreover, over 3,000 patents from 1970 to 2019 were reviewed with the search term ‘“microneedle” AND “insulin”’ to understand the current status of the field. In conclusion, the reporting of early stage microneedle research demonstrated a lack of consistency relating to the translational factors addressed. Additionally, a more rational design, based on a patient-centred approach is required before microneedle-based delivery systems can be used to revolutionise the lives of people living with diabetes following regulatory approval.
Introduction
The recent increase of research in the field of microneedle technology presents the opportunity to address the shortcomings of subcutaneous injections and transdermal patches. Considered as a hybrid between the hypodermic needle and the transdermal patch, microneedles are biomedical devices that consist of arrays of micro-projections on a supporting base, with a height in the range of 250 to 1000 µm (Sabri et al., 2019). Upon insertion, the formation of aqueous channels across the stratum corneum allows both small drug molecules and large macromolecules to enter into and across the skin (Kirkby et al., 2020). Importantly, a notable advantage of all microneedles from the patient’s perspective is the painless and minimally invasive application of the device to the skin. Despite a significant amount of research and microneedle devices becoming commonplace in the cosmetic sector, there remains significant barriers preventing microneedle devices from being approved for medical use (Kirkby et al., 2020).
One disease that has garnered considerable attention in microneedle research is diabetes mellitus. Diabetes is a complicated and debilitating illness, characterised by a partial or complete loss in the ability of the β-cells in the Islets of Langerhans, within the pancreas, to produce a suitable quantity of insulin to effectively regulate blood glucose concentration (American Diabetes Association, 2004). This presents immediate, dangerous risks for patients, such as diabetic ketoacidosis (DKA), alongside several severe long-term effects (Nyenwe and Kitabchi, 2016). Long-term effects are often categorised into macrovascular diseases, such as cardiovascular disease (CVD), cerebrovascular and peripheral vascular disease (PVD), and microvascular diseases, such as retinopathy, nephropathy and neuropathy (Nathan, 1993).
For many patients with a diagnosis of Type 1 Diabetes Mellitus (T1DM), multiple daily injections of insulin is the standard treatment option to effectively manage the condition (The Diabetes Control and Complications Trial Research Group, 1993). Compliance to these treatment regimens can be low, in part due to the use of traditional hypodermic needles, highlighting the potential for the clinical translation of microneedle technology (Peyrot et al., 2010).
In this review, the clinical translation of microneedle devices, using diabetes mellitus as a case study, is explored. Patents and publications for insulin-loaded microneedle devices will be critically evaluated in order to elucidate the steps which should be taken to enhance the chances of successful clinical translation. This review will be of value to those researching within the field of microneedle technology, particularly with a focus on diabetes mellitus, alongside clinicians who wish to understand more about the advantages and downfalls of microneedle technology.
Download the full study as PDF here The clinical and translational prospects of microneedle devices, with a focus on insulin therapy for diabetes mellitus as a case study
or read it here
For the creation of microneedles several excipients are mentioned in the study: Calcium phosphate, Maltose, Trehalose, chitosan, starch, PVA, PVP, PLGA
Fiona Smith, Akmal H. Sabri, Matthew Heppel, Ines Fonseca, Faz Chowdhury, Karmen Cheung, Stephen Willmor, Frankie Rawson, Maria Marlow, The clinical and translational prospects of microneedle devices, with a focus on insulin therapy for diabetes mellitus as a case study, International Journal of Pharmaceutics, Volume 628, 2022, 122234, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.122234.

