Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration
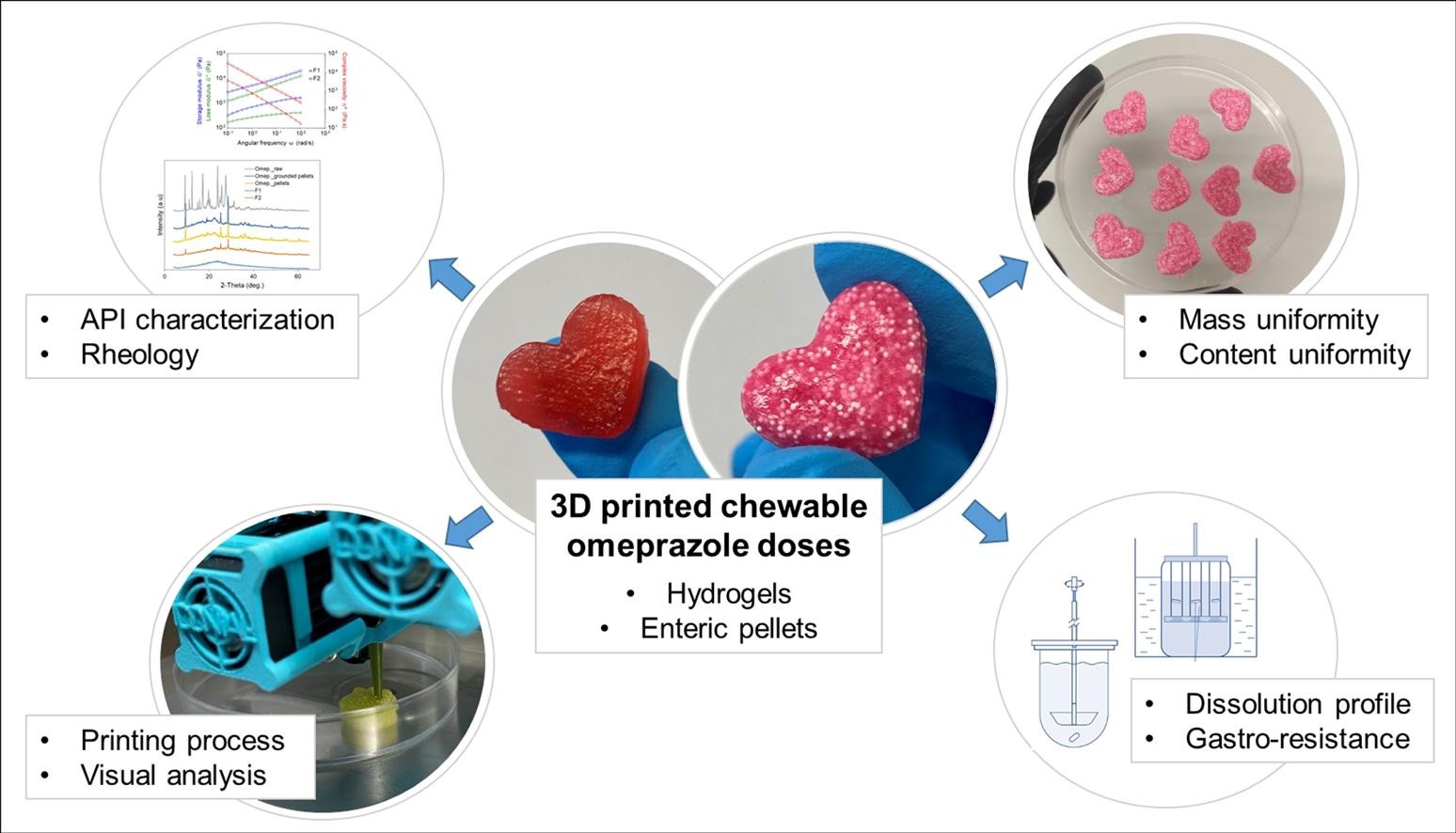
The production of 3D printed pharmaceuticals has thrived in recent years, as it allows the generation of customised medications in small batches. This is particularly helpful for patients who need specific doses or formulations, such as children. Compounding pharmacies seek alternatives to conventional solid oral doses, opting for oral liquid formulations. However, ensuring quality and stability, especially for pH-sensitive APIs like omeprazole, remains a challenge. This paper presents the application of semi-solid extrusion 3D printing technology to develop patient-tailored medicinal gummies, with an eye-catching appearances, serving as an innovative omeprazole pharmaceutical form for paediatric use. The study compares 3D printing hydrogels with dissolved omeprazole to hydrogels loaded with gastro-resistant omeprazole pellets, a ground-breaking approach.. Gastro-resistance and dissolution profiles were studied using different methods for better comparison and to emphasize the significance of the assay’s methodology. Both developed formulas exhibit proper rheology, good printability, and meet content and mass uniformity standards. However, the high gastro-resistance and suitable release profile of 3D printed chewable semi-solid doses with enteric pellets highlight this as an effective strategy to address the challenge of paediatric medication.
2.1. Materials
Omeprazole (CAS no. 73590–58-6) and gelatin (CAS no. 9000–70-8) were purchased from Merck KGaA, Darmstadt, Germany. Micronized omeprazole for pellet coating was given from Esteve Química, Barcelona, Spain. Xanthan gum (CAS no.11138–66-2), Lactose monohydrate (CAS no. 10039–26-6), sodium lauryl sulphate (CAS no. 151–21-3), titanium dioxide (CAS no. 13463–67-7), Talc (CAS no. 14807–96-6) and purified water (CAS no.7732–18-5) were purchased from Fagron Ibérica SAU, Terrassa, Spain. Carboxymethyl Cellulose (CAS no. 9004–32-4) and glycerol (CAS no. 56–81-5) were purchased from Guinama S.L.U, Valencia, Spain. Carrageenan (Gelification Iota®) was acquired through Guzmán Gastronomía SL, Barcelona, Spain. Lemon essence (Aroma de limón, Dr. Oetker Ibérica, Barcelona, Spain), lemon juice (Limón exprimido Hacendado, JR Sabater S.A., Murcia, Spain), liquid sweetener (Edulcorante de mesa líquido Hacendado, Jesús Navarro S.A., Alicante, Spain), sodium bicarbonate (Bicarbonato sódico Hacendado, Jesús Navarro S.A., Alicante, Spain) and food coloring (Colorante alimentario Vahiné®, McCormik España, Sabadell, Spain) were purchased from a local convenience store. Vivapur® MCC spheres were purchased from JRS Pharma Gmbh & Co. KG, Rosenberg, Germany. Hydroxypropyl methyl cellulose (CAS no. 9004–65-3) and hydroxypropyl cellulose (CAS no. 9004–64-2) were purchased from Shin-Estu Chemical Co., Ltd., Tokyo, Japan. Eudragit® L-30 D-55 was purchased from Evonik Corp., Barcelona, Spain. Triethyl citrate (CAS no. 77–93-0), disodium dihydrogen phosphate (CAS no. 7558–79-4), sodium dihydrogen phosphate (CAS no. 7558–80-7), sodium tetraborate decahydrate (CAS no. 1303–96-4), tribasic sodium phosphate dodecahydrate (CAS no. 10101–89-0), sodium hydroxide (CAS no. 1310–73-2), disodium hydrogen phosphate 12-hydrate (CAS no. 10039–32-4), hydrochloric acid 5 M (CAS no. 7647–01-0) and ethanol 96 % (CAS no. 64–17-5) were purchased from PanReac Química S.L.U., Barcelona, Spain.
Download the full article as PDF here: Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration
or read it here
Khadija Rouaz-El Hajoui, Helena Herrada-Manchón, David Rodríguez-González, Manuel Alejandro Fernández, Enrique Aguilar, Marc Suñé-Pou, Anna Nardi-Ricart, Pilar Pérez-Lozano, Encarna García-Montoya, Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration, International Journal of Pharmaceutics, Volume 643, 2023, 123289, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123289.

