Development, evaluation and recent progress of ocular in situ gelling drug delivery vehicle based on poloxamer 407
Abstract
The fabrication of an effective in situ gelling ophthalmic drug delivery vehicle is recently received enormous interest to overcome the demerits of conventional ocular solutions and achieve desired therapeutic efficacy. Poloxamer 407 and poloxamer 188 are among the most commonly used poloxamers in ocular drug delivery due to thermoreversible gelation property, high solubility in water and transparent gel formation capacity which does not hamper normal vision. In this review, we have mostly focused on the thermogelation properties of poloxamer 407 as well as various methods to evaluate the poloxamer based ophthalmic in situ gel for their potential applications in ocular drug delivery and have also discussed about different strategies to improve thermogelation properties and drug release behavior of poloxamer 407 based ophthalmic formulations. We have also discussed here that the use of poloxamer 407 as thermoreversible in situ gel in colloidal drug carrier systems has proved to be an effective strategy for increasing penetration of drugs, enhancing therapeutic action, and reducing drug release rate.
Introduction
Eye is seems to be the most simple and easily accessible organ for tropical treatment. But it hardly accepts any foreign particle primarily by eyelids and tear flow and secondly due to the protective mechanism of cornea. When any foreign materials or medication is applied on the surface of the eye, it is wiped out quite quickly by rapid tear flow. Owing to these anatomical and physiological obstacles of the eye, the field of ocular drug delivery is considered as the most demanding and daunting pursuits of diverse drug delivery routes to the pharmaceutical scientist for previous 10–20 years. Drug is applied on ocular surface either to treat the external eye for various sorts of diseases or to offer intraocular treatment through the cornea. Ocular medicine in drop form is most conventional doses form of ophthalmic medication for the treatment of maximum eye sicknesses such as conjunctivitis, blepharitis, keratitiessicca, glaucoma or uveitis. In the market, about 90% of the ocular medicines are available as drop form [1]. But when this ocular dosage form is applied to eye surface, very fast washing out of a large portion of the imparted drug takes place from the precorneal region because of several loss factors like lachrymal secretion, nasolacrymal drainage, blinking reflex, limited contact period in the cul-de-sac, systemic conjunctiva inclusion and low corneal epithelial membrane penetration. Due to these physiological as well as anatomical obstruction, just a little portion (effectively 1% or less) of the applied dose is ocularly consumed and this leads to inadequate bioavailability of medication. Now the goal is to fabricate a therapeutic framework which provides an optimum concentration of the drug for long term at the active site to achieve desired therapeutic effect. Thus, it is rather needful to instill eye drops very frequently or higher concentration of the drug containing formulation but these may be the reason of adverse toxicity and corneal cell harm.
Other form of delivery systems such as viscous solutions, preformed hydrogels, ointments, polymeric inserts, suspensions, etc., have been studied with the aim of upgrading bioavailability of the eye medication and slowing down drug diffusion rate [2]. Viscous solutions are more profitable than eye drops as it offers prolonged residence time, however it cannot present effective improvement in ocular bioavailability. Suspensions are typically designed for the application of feebly dissolvable drugs, although their particle size restricts uses because it prompts aggravation and grittiness on application [3]. Ointments are not suitable owing to their greasy nature, obscuring of vision and infrequent aggravation [4]. The application of preformed hydrogel faces several drawbacks. In this case drug loading happen through inaccurate and irreproducible way and high thickness of the gel causes blurred vision of the patient, malted eyelids, and lachrymation which limits their uses as ocular medication device [5,6]. Ocular inserts provide perfect dosing of medications, yet a few burdens like patient incompliance, complications during self-insertion, remote molecule sensation or inadvertent loss from the eye prompts trouble for application [7]. From the above discussions, it can be concluded that the existing conventional doses form of ocular medication are not effective to treat extreme ocular diseases, therefore cannot be applied widely for ocular drug delivery. In ocular treatment, the liquid drop form is most appropriate in respect of patient acceptability.
- 1. Sufficient corneal infiltration.
- 2. Ease in administering.
- 3. Ideal in drop structure.
- 4. Decreased instillation frequency.
- 5. Permissible to Patient.
- 6. Increasing ocular medication assimilation through extended contact with corneal tissue
- 7. Least toxic and side effect.
- 8. Continued drug discharge rate.
- 9. Non-irritative and comfortable forms (viscous solution should not promote lachrymal discharge and rapid blinking of eye).
- 10. The vision should not to be blurred.
- 11. Suitable rheological features and concentration of viscous system.
In situ hydrogel based framework can be the substitute by executing all of these criterions.
Hydrogels are made of hydrophilic polymeric network having three dimensional cross-links. It can swell drastically by attracting extremely huge quantity of water without disintegrating into it [[9], [10], [11], [12], [13]]. Hydrogels have numerous applications particularly in biomedical and pharmaceutical field because of their exceptional features [[14], [15], [16]]. Such unique properties are attributed to their capacity to hold enormous amount of water under physiological conditions, rubber like softness and consistency, and lower interfacial stress with water and biological fluid. Attributable to these properties, hydrogels fundamentally behave the same as living tissue. It can be implanted or inoculated in the body creating least amount of cell adherence and sensitivity [[17], [18], [19], [20], [21], [22]]. Hydrogels can therefore be utilized as contact lenses, biosensors membranes, artificial heart lining, artificial skin materials, and drug delivery vehicles [[14], [15], [16],23,24]. Polymeric hydrogels are very useful component for the preparation of ophthalmic drug delivery vehicle to extend the contact period in precorneal area and, hence, improve ocular bioavailability. It is possible to insert drug into hydrogel either through incubating the preformed cross-linked gel in concentrated solution of drug or by encapsulating in situ. In the subsequent situation drug inserting and cross-linking process takes place concurrently on the surface of eye with certain physiological parameters like temperature, pH or some ions [25,26]. This specific type of drug delivery hydrogel system is called in situ gel forming system. The administration of ophthalmic preparation is very easy because it is applied conventionally in the drop form and undergoes sol to gel transition on the ocular surface, thus accurate quantity of drug is possible to administer [27] and as their residence time can be elongated and therefore can upgrade the drug’s residence period at the point of action. It provides improved drug bioavailability and maximizes compliance of patient when contrasted with ordinary ophthalmic vehicles of drug [5,[28], [29], [30], [31]]. Several polymer based in situ gel formulations have been exploited to deliver a varieties of drug for therapeutic benefits, for example, Ofloxacin, Ciprofloxacin, Gatifloxacin, Timol, Ketorolac Tromethamine, Pilocarpine hydrochloride and so on which highlight the relevance of in situ gelling products as a powerful ophthalmic drug delivery vehicle in future [5,[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]. Some polymer-based in situ gel drug delivery systems are approved for market which is shown in Table 1.
In situ gelling framework has pseudo plastic flow behavior that is exceptionally useful in limiting obstacle with eye blinking. Drug can be effectively filled into the polymeric in situ gel matrix by dissolving or dispersing, which upon application onto corneal surface experience gelation, responding physiological conditions and form gel having viscoelastic nature due to modification in polymer conformation [28,29,39,47].
Among the various in-situ polymer hydrogel systems, thermo responsive hydrogels are mostly studied class for the application of controlled release of drug because it is naturally variable and easy to control. Modification of mechanical behavior as well as drug release features of this type of hydrogel take place because of the variation of temperature of external environment [52,53]. This type of hydrogel is potent to swell and shrink/deswell when surrounding fluid temperature is modified [54]. The general characteristics of thermo sensitive hydrogels are controlled by both hydrophobic component and hydrophilic domain present in the structures and the occurrence of temperature response is consequence of the exquisite balance between the hydrophobic segment and hydrophilic component of any single polymer molecule [55,56]. Now, the interaction of hydrophilic as well as hydrophobic segment with the water molecule is changed with the temperature resulting in the change of solubility of cross-linking network structure of hydrogel and gelation process is observed as a consequence of that [57]. Hydrogel undergoes this phase transition either at lower critical solution temperature (LCST) or at the upper critical solution temperature (UCST) [58,59]. The polymer is soluble in water under the LCST or above the UCST, on contrary it begins to shrink and become insoluble above the LCST or below the UCST and then gel formation takes place due to resulting hydrophobic aggregation. The inherent LCST or UCST of a hydrogel can differ if the proportion of hydrophilic to hydrophobic segment of the polymer is altered [55]. Thermosensitive hydrogel is categorized into three groups which are negatively thermo-sensitive, positively thermo-sensitive and thermo reversible gel [60,61].
Negatively thermo-sensitive hydrogel has LCST and when temperature reaches LCST it contracts and bellow the LCST it swells [62,63]. LCST systems are fundamentally significant for release of drug, specifically proteins, in sustained way [[64], [65], [66]]. This hydrogel represents ‘on-off’ release pattern of drug from gel matrices and lower temperature promote ‘on’ where as a high temperature favors ‘off’ permitting pulsatile release design of drug [67,68]. A case of hydrogel system with a negative thermosensitivity is PVP/PNIPAAM (polyvinylpyrrolidone/poly(N-isopropylacrylamide)) based hydrogel that is used in drug delivery [69].
Positively thermosensitive hydrogels have UCST, on cooling this type of hydrogel shrinks bellow the UCST and is soluble in water above UCST. There are different hydrogels made of interpenetrating polymer networks, which have positive thermosensitive gelation property. When LCST of polymer solutions ranges between ambient and physiological temperature, then these systems can be utilized for injectable hydrogel preparation. Such a system is liquid in nature & flow freely at room temperature, hence can be easily injected into the body cavity where ever it is required, but form gel after instillation into the body. In recent times, new arrangement of triblock copolymer is designed which is biodegradable. This polymer consists of PEG-PLGA-PEG (PEG: Polyethylene glycol, PLGA: Poly (lacto-co-glycolic acid)) or PLGA-PEG-PLGA which is fabricated for sustained injectable drug delivery [66,70]. Thermally reversible hydrogel has the ability to experience gelation rather than swelling – deswelling interchange. This type of hydrogel does not have chemically cross-linked polymer chain although they have the same structure and content as that of the negatively and positively temperature responsive hydrogel. Pluronics and Tetronics, obtained from poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide), are the most renowned synthetic polymer having thermoreversible gelation property and utilized vastly in the drug delivery area [55,71,72]. Xyloglucan, a natural polymer, also have thermoreversible gelation property [5].
Poloxamers are the most renowned stimuli-responsive polymers that exhibit sol-gel transition behavior in response to temperature. Poloxamer block copolymers were primarily projected during the 1950s (by BASF, NJ, USA) and from that point forward these have been applied in pharmacy and biomedical field [[73], [74], [75], [76], [77]]. These are enlisted in the US and European Pharmacopoeia [77].
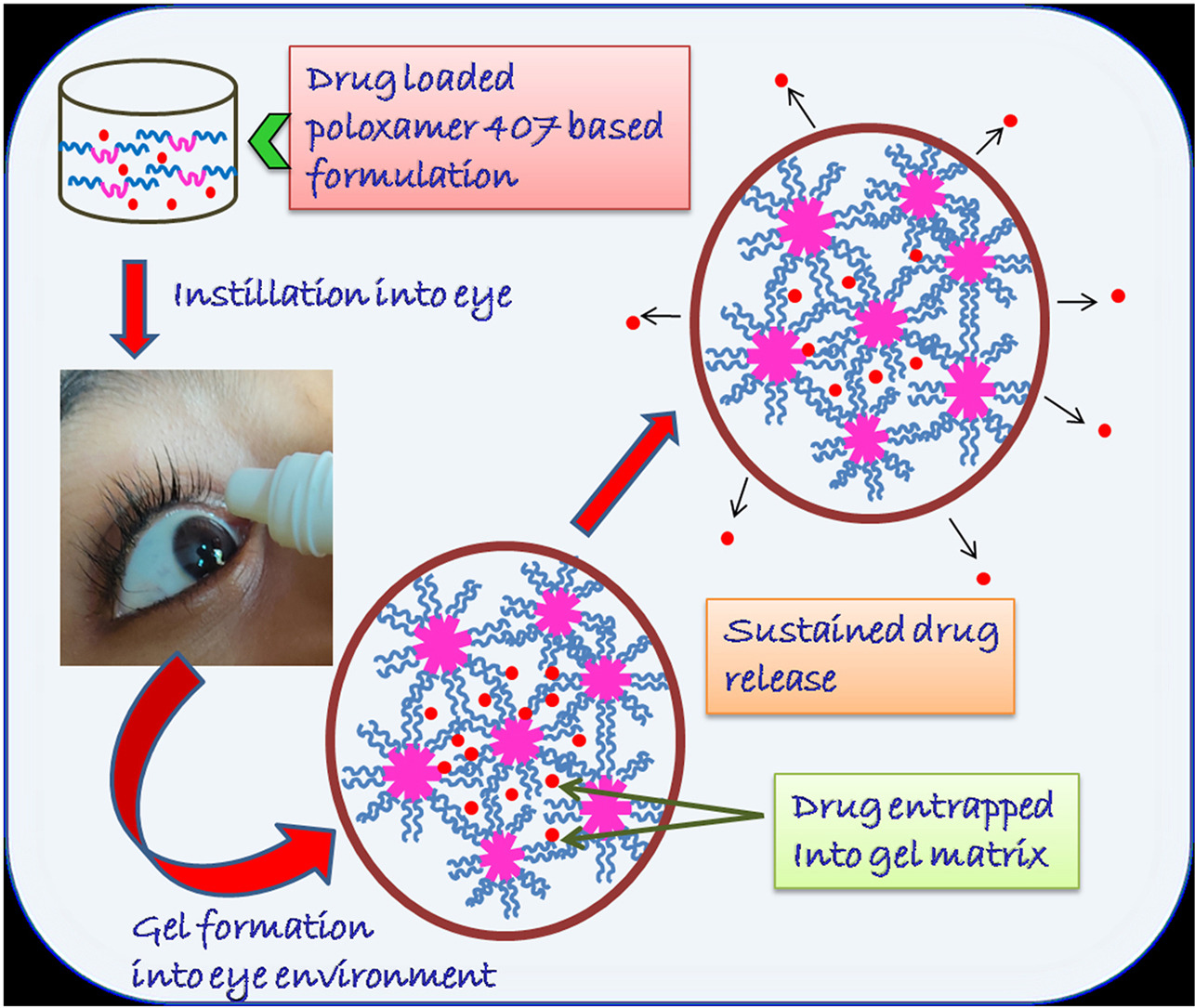
Poloxamer 407 (P407) and poloxamer 188 (P188) are among the most commonly used poloxamers in ocular drug delivery. P407 is highly soluble in water and form transparent gel, so does not hamper normal vision. Strength and viscosity of P407 gels improves with increasing total poloxamer content in the solution which is reported by Baloglu et al. [78]. The aqueous solution of P407 have sheer thinning behavior, it may be utilized very securely without harming the ocular tissues. Several in vitro, ex vivo, and in vivo trial of biocompatibility of P407 proved their non toxicity on corneal tissues [79,80]. All these reasons make P407 most frequently utilized polymers as ophthalmic drug delivery vehicle. Various examinations additionally demonstrated that, the integrity of P407 gel stay unaffected against steam sterilization and autoclave sterilization at 120 °C for 15 min at 1 bar pressure [81,82]. So, it is feasible to set up sterile formulations for ophthalmic routes by utilizing this polymer [83]. A few P407 based in situ gelling ophthalmic formulations are already available in market and some formulations are in clinically trial phase mainly for the treatment of glaucoma and bacterial conjunctivitis as shown in Table 2 [84].
In this review, we have mostly focused on the chemical structure and thermogelation properties of P407 as well as various methods to evaluate the P407 based ophthalmic in situ gel for their potential applications in ocular drug delivery and have also discussed about different strategies to improve thermogelation properties and drug release behavior of ophthalmic formulations based on this polymer.
Read more here
Mitali Dewan, Arpita Adhikari, Rathin Jana, Dipankar Chattopadhyay, Development, evaluation and recent progress of ocular in situ gelling drug delivery vehicle based on poloxamer 407, Journal of Drug Delivery Science and Technology,
Volume 88, 2023, 104885, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.104885.
Read more on “Ocular Delivery” articles here:
- Controlled release, chitosan-tethered luteolin phytocubosomes; Formulation optimization to in-vivo antiglaucoma and anti-inflammatory ocular evaluation
- MeltSerts Technology (Brinzolamide ocular inserts via hot-melt extrusion): QbD-steered development, molecular dynamics, in vitro, ex vivo and in vivo studies
- Development of Osthole-Loaded Microemulsions as a Prospective Ocular Delivery System for the Treatment of Corneal Neovascularization: In Vitro and In Vivo Assessments