Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation – Where Are We Now?
Abstract
Hot-melt extrusion (HME) is a globally recognized, robust, effective technology that enhances the bioavailability of poorly soluble active pharmaceutical ingredients and offers an efficient continuous manufacturing process. The twin-screw extruder (TSE) offers an extremely resourceful customizable mixer that is used for continuous compounding and granulation by using different combinations of conveying elements, kneading elements (forward and reverse configuration), and distributive mixing elements. TSE is thus efficiently utilized for dry, wet, or melt granulation not only to manufacture dosage forms such as tablets, capsules, or granule-filled sachets, but also for designing novel formulations such as dry powder inhalers, drying units for granules, nanoextrusion, 3D printing, complexation, and amorphous solid dispersions. Over the past decades, combined academic and pharmaceutical industry collaborations have driven novel innovations for HME technology, which has resulted in a substantial increase in published articles and patents. This article summarizes the challenges and models for executing HME scale-up. Additionally, it covers the benefits of continuous manufacturing, process analytical technology (PAT) considerations, and regulatory requirements. In summary, this well-designed review builds upon our earlier publication, probing deeper into the potential of twin-screw extruders (TSE) for various new applications.
Download the full article as PDF here: Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation—Where Are We Now?
Introduction
The pharmaceutical industry is undoubtedly intrigued by hot-melt extrusion (HME) technology, as evidenced by the substantial increase in patents and publications. HME is rapidly becoming the preferred choice in the pharmaceutical industry due to its solvent-free and eco-friendly approach to continuous manufacturing [1, 2]. HME has proven highly effective in improving the solubility and dissolution of poorly water-soluble drugs by creating amorphous solid dispersions. Additionally, it is an essential tool for producing innovative pharmaceutical products [3, 4]. During the process, the active pharmaceutical ingredient is mixed at a molecular level with thermoplastic binders and/or polymers while passing through high-temperature counter-rotating or corotating screw elements. This meticulous molecular mixing process transforms the active pharmaceutical ingredient into an amorphous form, enhancing the dissolution rate [5, 6]. HME offers numerous advantages over traditional methods, as previously highlighted in part of this review titled “Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation in AAPS PharmSciTech, 2016” [6].
In our earlier published review, the authors explored the intricacies of HME technology, discussing the various types of process technology and equipment, types of screw extruders, principles of operation, and raw materials used. Our insights with multiple case studies back it up and explore the diverse range of pharmaceutical applications of HME. Furthermore, the article highlighted the advantages of HME as a continuous pharmaceutical manufacturing process while also addressing the challenges of scaling up and optimizing the process using QBD approaches.
Extruders are designed as single-screw, twin-screw, or multi-screw extruders, and any extruder must meet regulatory standards for pharmaceutical dosage manufacturing. The twin-screw extruders (TSE) offer superior performance in the pharmaceutical manufacturing sector, as discussed in our previous review. This well-constructed review builds upon our earlier publication, delving deeper into the potential of TSE for various new applications. These applications include the creation of dry powder inhalers, TSE as effective drying equipment, nanoextrusion, cocrystals, complexation, and solid dispersion. Additionally, this review covers various types of twin-screw granulation and equipment modifications and provides relevant case studies. The section on HME as a continuous manufacturing process has been significantly expanded to include scale-up considerations, process analytical technology (PAT) considerations, and valuable insights into marketed products.
Tools Used to Prepare the Review
Various search engines, such as Google Scholar, PubMed, and ResearchGate, primarily focused on Google Scholar due to its widespread usage, were employed to explore original research and review articles. The broad search strategy encompassed various relevant articles, ensuring comprehensive coverage. Specific criteria were established to select papers, focusing on peer-reviewed articles with substantial citations from peer-reviewed journals. The search utilized keywords such as “Twin Screw Extrusion,” “Twin screw granulation,” “Hot-Melt Extrusion,” “Twin screw extrusion pharmaceutical applications,” “Hot-Melt extrusion + Scale-up,” “HME coupled fused deposition modeling 3D printing,” “HME + continuous manufacturing,” “HME + PAT tools,” etc. Additionally, a supplementary search was conducted in review papers and Google patents to identify formulations manufactured using Hot-Melt Extrusion that were marketed. Manual titles and abstracts were screened to identify the most relevant information and essential case studies.
Pharmaceutical Applications of Twin-Screw Extruders
Significant progress has been observed in developing novel applications using TSE technology, which is also reflected by the increased number of recent publications and patents in this area. Several novel applications of TSE are discussed in the following section.
TSE as a Mixer for Dry Powders for Inhalation
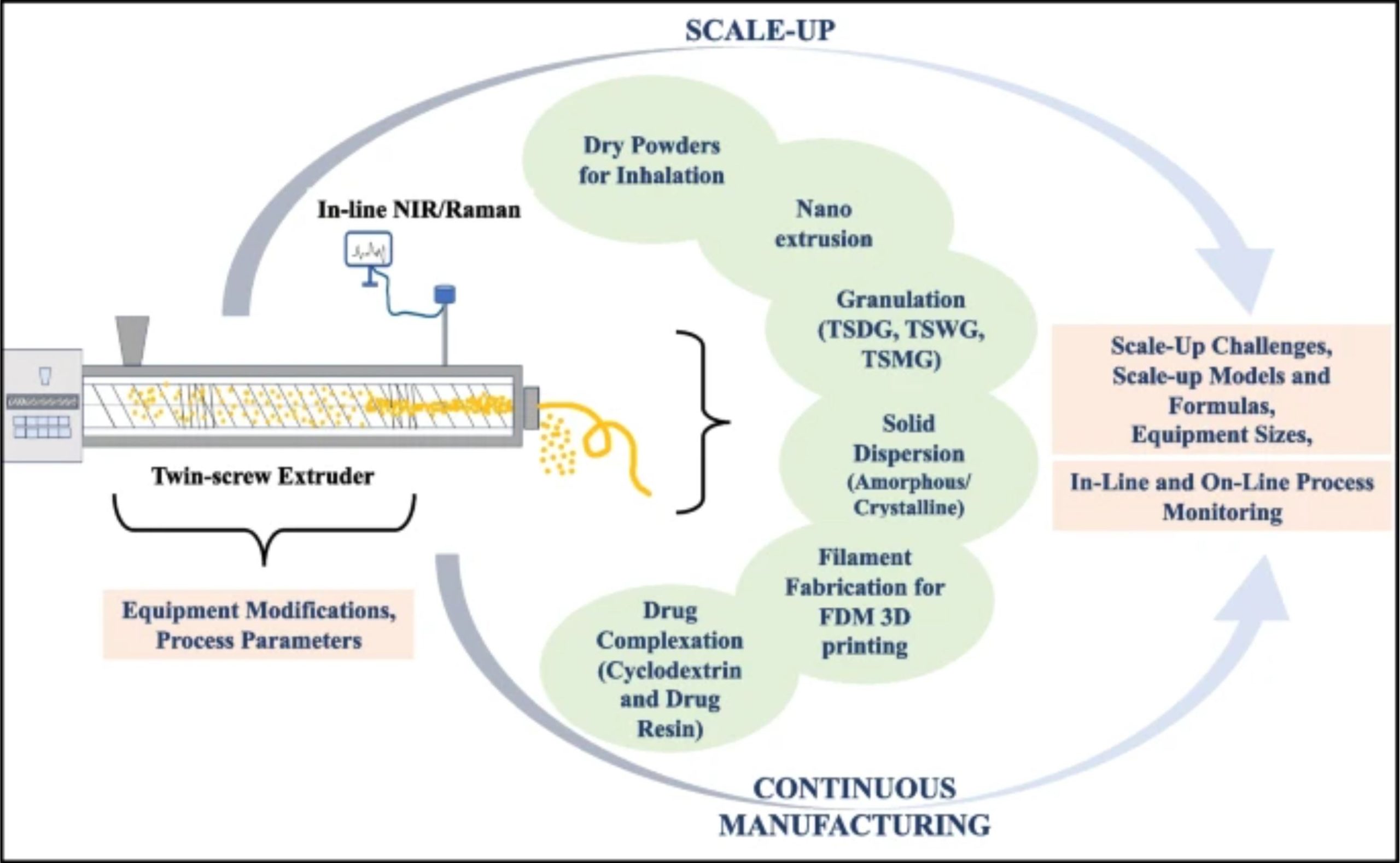
Dry powder inhalers have gained popularity as a delivery method due to their numerous advantages over the years. In this system, a larger quantity of coarser excipient particles is mixed with a smaller portion of a potent drug of relatively finer particles [7]. Moreover, the drug’s performance and lung delivery rely on adhesion forces with the excipient. Therefore, achieving a homogenous blend uniformity is critical in developing dry powder inhalation dosage forms [7]. Different techniques have been developed to achieve a uniform distribution of the drug in the final dosage form. However, these methods may experience batch-to-batch variations due to inadequate mixing. Using HME as a mixer for dry powders can overcome this challenge and enable continuous manufacturing by dispensing the blend into final containers with proper setup [8]. Twin-screw extruders offer the opportunity to continuously manufacture dry powders for the inhalation [8]. Recently, Ren et al. used a twin-screw co-rotating extruder as a continuous mixer for blending powders for inhalation [9]. The authors aimed to reduce batch-to-batch variations when blending in tumble or high-shear batch mixers. The screw profile can be seen in Fig. 1a.
The extruder was fed using two volumetric twin-screw feeders, one containing the API and the other containing the excipients. Then, both streams were blended in the extruder. The combing mixing elements blended the powder streams with high efficiency due to the axial slits that can be observed in Fig. 1. The extruder-blended powders had similar aerosol performance to those blended using high-shear batch mixing, and in the extruder, the mixing was done in less than 2 min [9]. As mentioned, the authors used an intermeshing twin-screw co-rotating extruder, a “self-wiping” setting, that has traditionally demonstrated reduced losses during production [10, 11]. In their following research article, the authors evaluated the effect of employing different mixing elements and processing parameters on the uniformity and aerosol performance of the powders for inhalation.
Among the findings, the authors demonstrated that the use of 30° kneading elements was able to obtain better homogeneity than using the combing mixing elements employed in their previous publication [9]. Regarding aerosol performance, there were no differences between combing mixing and the 30° kneading elements for all feed rates/screw speeds tested until reaching too high specific energies (> 10 g/min and 500 rpm). The behavior of powder mixing on the twin-screw mixer is interesting, especially when examining differences between segregating and non-segregating powders. According to Pilcer et al., non-segregating powders may require a longer mixing time to achieve the desired results. On the other hand, segregating powders tends to stick together in clumps, and a specific mixing time is necessary to break them apart for optimal results [12]. This information highlights the importance of understanding the properties of different types of powders and tailoring the mixing process accordingly.
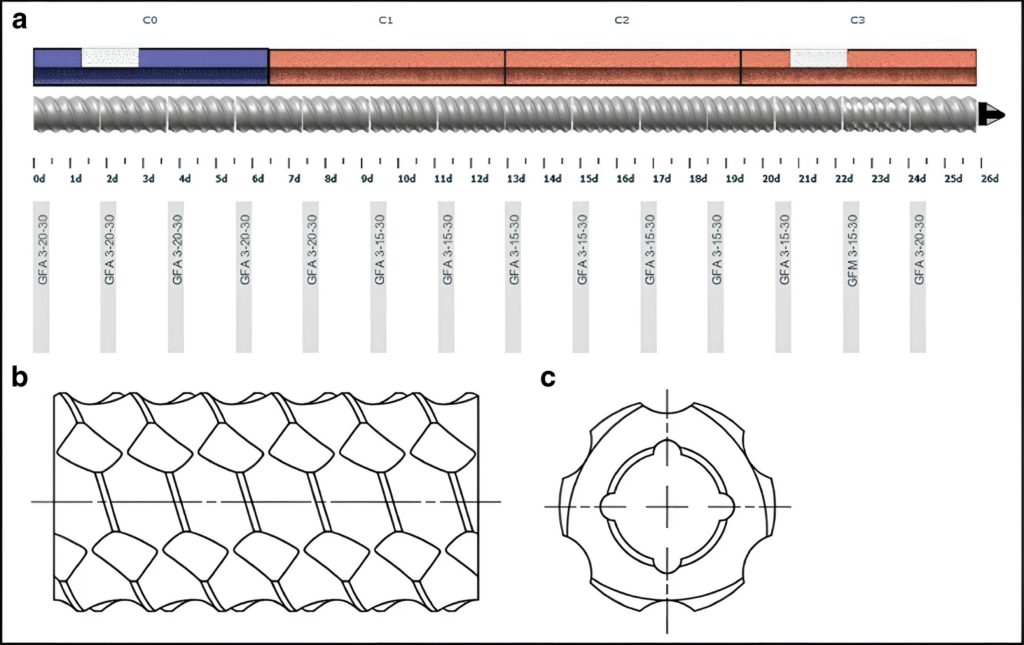
This approach using TSE for mixing the different components can effectively avoid a unit operation to make pre-blends before the extrusion process by feeding formulation components separately into the extruder (Fig. 2).
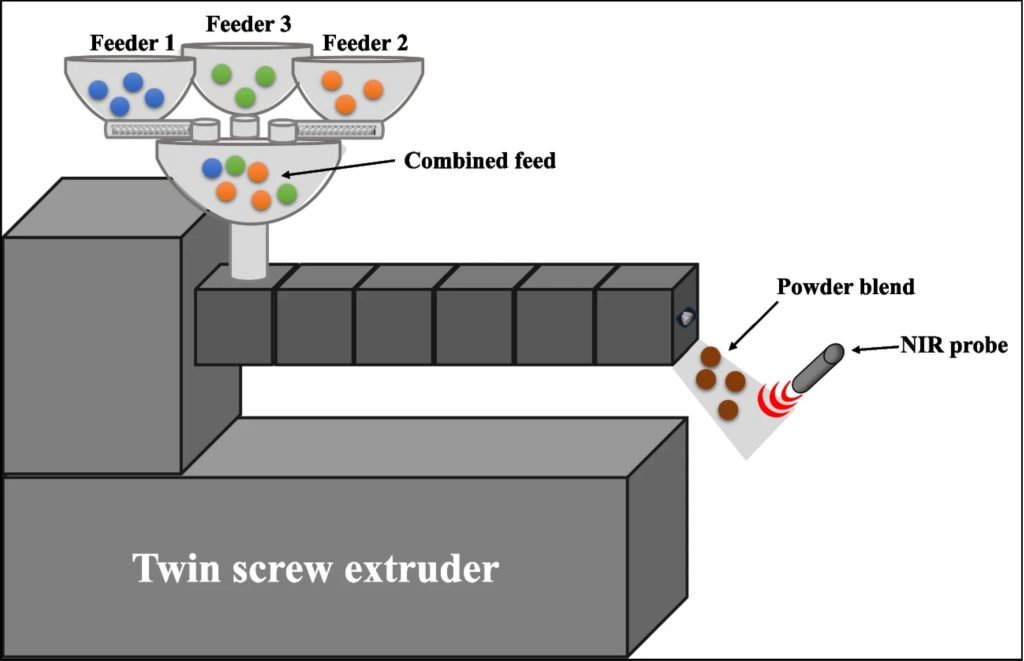
Twin Screw Extruders as Drying Equipment
Drying the materials is critical in commercial manufacturing, and it requires thermal energy. In the manufacturing of solid dosage forms, drying the API is a crucial step. If drying is not controlled, inappropriate morphology of particles or an undetermined polymorph state can occur. During the drying process, several phenomena occur, such as agglomeration, attrition, (re)-crystallization, and redissolution. Agglomeration produces larger particles, which leads to slower dissolution, while attrition produces smaller particles, resulting in poor flowability [13]. The drying process reduces particle size via shearing or induced stresses caused by particle collisions. It also influences the particle size distribution of the final product. Additionally, the residual moisture content in the final product causes instability of the active pharmaceutical products. Several techniques have been employed to dry the material, such as tray drying, fluid-bed systems, spray drying, and paddle drying. However, drying smaller particle-size material with poor flowability using the existing technologies is very challenging. Therefore, hot-melt extrusion can address these constraints along with continuous manufacturing. The twin-screw extruder is a highly effective drying technology that outperforms other methods like fluidized bed dryers. It ensures precise temperature control and can dry materials below their glass transition temperature, leading to minimal/no particle size and quality changes. Additionally, the extruder’s residence time has little impact on reducing particle size [14].
In the pursuit of the transition from batch manufacturing toward continuous manufacturing, wet granulation is particularly challenging because it requires several unit operations [15]. A twin-screw extruder can easily combine the blending using a multi-feeder approach, as shown in Fig. 3, and granulation steps into one unit operation [16]. However, the drying unit operation is more challenging for an extruder [15]. In recent years, several research groups have envisioned using a twin-screw extruder to simultaneously perform wet granulation and drying in one single and continuous unit operation.
Meena et al. (2017) optimized extruder parameters and were able to produce acetaminophen granules with moisture of 0.1% w/w. These granules did not require milling or sieving before tableting. Schmidt et al. (2018) studied in depth the drying capabilities of twin screw granulation, operating at higher temperatures and with higher amounts of water than the study reported by Meena et al. (2017) [16]. The authors compared the granules produced by a corotating Pharma 11 mm extruder with a fluidized bed dryer. The authors studied variables such as mass flow rate, L/S ratio, screw speed, and temperature in different extruder zones. One of the most critical findings is that screw speed has a significant impact on controlling heat transfer by modifying the residence time of the material in the extruder. Additionally, the study found differences in disintegration when comparing the granules from in-barrel-drying and fluidized-bed drying [16].
In a subsequent research article, Schmidt A et al. (2018) aimed to perform wet granulation and drying using an API suspension instead of solid feeding. They aimed to reduce the unit operations needed to transition from primary to secondary manufacturing. The authors demonstrated that it is feasible to combine API crystallization and drying (primary manufacturing) with wet granulation and drying (secondary manufacturing), performing several operation units in one step [13]. Kreimer et al. (2018) employed a twin-screw extruder to dry a crystallized product during primary manufacturing with a similar purpose because improper drying can lead to changes in particle size distribution and morphologic changes that can negatively impact further manufacturing of an API [17].
It is known that process validation must be pursued in each production scale, and usually, the optimized parameters from a laboratory scale are not transferrable to larger scales. Haser et al. (2023) claimed they were the first to achieve simultaneously wet granulation and drying in a single unit operation using a pilot scale twin-screw extruder (ZSE HP-18, American Leistritz Extruder Corp). In their barrel configuration, the authors tested different screw designs and the addition/position of vacuums in the vents, significantly impacting moisture removal. Additionally, the authors were able to monitor the process in-line using the NIR spectroscopy [18].
Read the full original article here
Excipient categories mentioned in the study: MCC, lactose, mannitol, Dicalcium Phosphate, HPMC, HPC, PVP
Patil, H., Vemula, S.K., Narala, S. et al. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation – Where Are We Now?. AAPS PharmSciTech 25, 37 (2024). https://doi.org/10.1208/s12249-024-02749-2

