Formulation development of methotrexate lipid-based nanogel for treatment of skin cancer
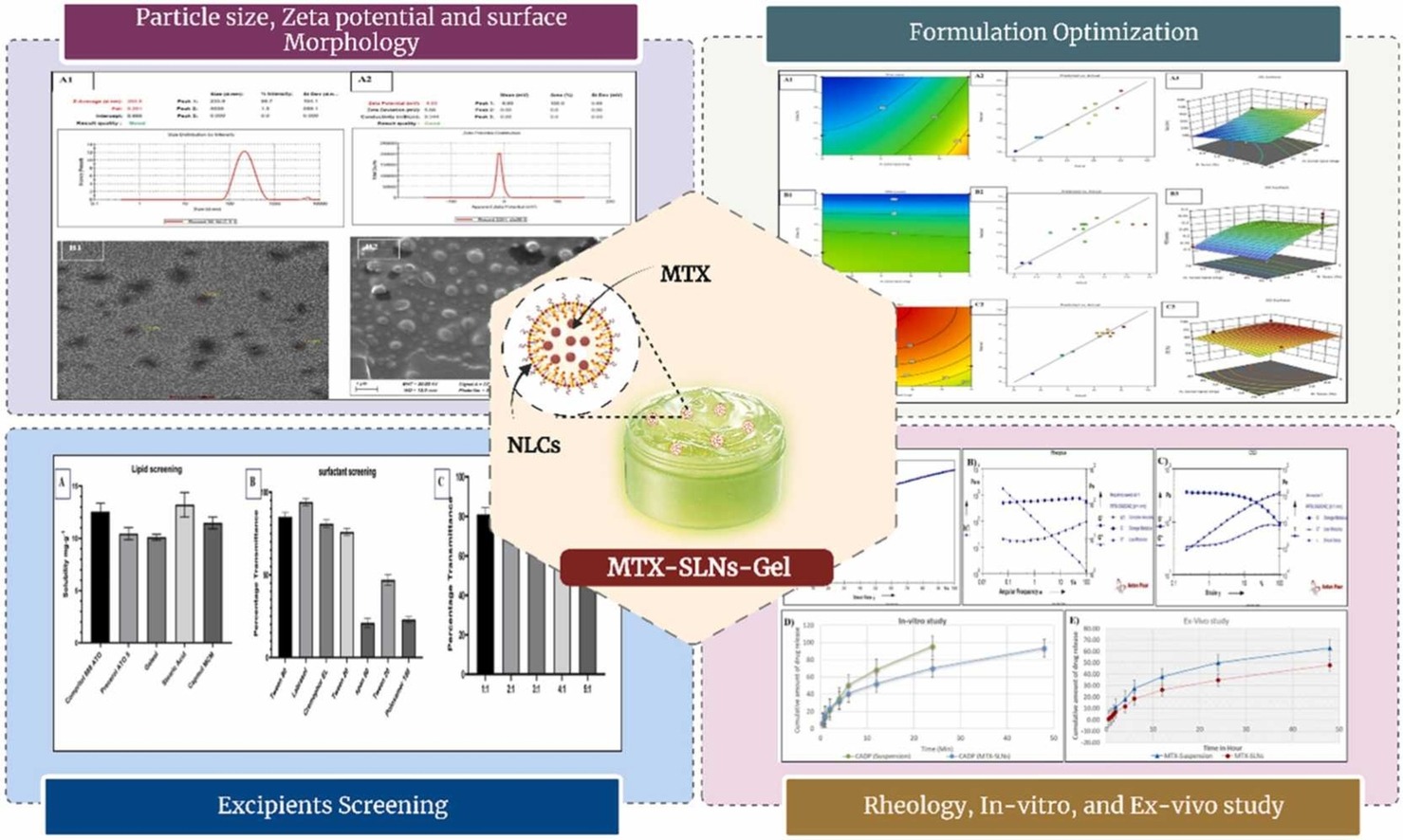
Skin cancer (SC) represents a prevalent malignancy worldwide, characterized by its high drug resistance and limited therapeutic efficacy with conventional treatments. Nanoparticles have emerged as promising vehicles for targeted drug delivery, aiming to overcome these challenges and enhance treatment outcomes. The solid lipid nanocarriers (SLNs) provide improved drug absorption, encapsulation, and controlled release. These SLNs can be customized to meet unique needs, allowing for enhanced targeting, distribution to specific sites, and profiles of sustained release.
This study was focused on the development and optimization of methotrexate loaded solid lipid nanoparticles (MTX-SLNs) for improving the drug delivery to the skin cancer therapy. The optimized MTX-SLNs exhibited a particle size of 203 nm, a zeta potential of -8.93 mV, entrapment efficiency of 87%, and drug loading of 62.92%. Subsequently, the optimized MTX-SLNs was incorporated into a gel system containing 1% Carbopol 934, which was further underwent comprehensive characterization, encompassing analysis of rheological properties, texture profile analysis, extrudability and spreadability, as well as uniformity of content. Notably, the dermatokinetic and confocal microscopy evaluations revealed excellent uptake and deposition of MTX-SLNs within the epidermal and dermal layers of the skin.
These observations were complemented by the computation of the area under the curve (AUC0-8hr), which demonstrated values of 245.035 and 702.59 for the epidermal and dermal layers, respectively. These findings underscore the tremendous potential of our MTX-SLNs gel formulation as a promising candidate for skin cancer treatment compared to the conventional topical gel therapies, exhibiting superior topical pharmacokinetics, nanometric size, high drug loading capacity, and enhanced efficacy.
Read more here
Materials
Methotrexate (MTX), Cremophor EL & RH40, Transcutol P, Poloxamer 407, Tween 80 & 20, Carbopol 934, were purchased from Sigma Aldrich, Bengaluru (India). Precirol®ATO-5, Geleol, Gelucire®50/13 & 44/14, Compritol®88, Labrasol®, and Capryol®90 were purchased from Gattefosse India Pvt Ltd, Mumbai, India, while Capmul® was purchased from ABITEC, IMCD (India).
Md. Abul Barkat, Nazeer Hasan, Mohd. Zaheen Hassan, Yahya I. Asiri, Arif Nadaf, Farhan J. Ahmad, Prashant Kesharwani, Formulation development of methotrexate lipid-based nanogel for treatment of skin cancer, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 133571, ISSN 0927-7757, https://doi.org/10.1016/j.colsurfa.2024.133571.
Read also our introduction article on Topical Excipients here:


