Solubility enhancing lipid-based vehicles for artemether and lumefantrine destined for the possible treatment of induced malaria and inflammation: in vitro and in vivo evaluations
Background
The lipid self-emulsifying system has been advanced as a promising delivery vehicle for improving the solubility and bioavailability of artemether and lumefantrine. However, the observed kinetic instability (propensity of lumefantrine to rapid crystallisation from nano-scale droplets) in aqueous acid has impelled some researchers to incorporate surfactants/solubilizers in the dissolution medium prior to dissolution studies. Thus, in our present work, we sought to prepare micro/large nano-scale (> 100 nm) and yet kinetically stable lumefantrine lipid self-emulsifying system (that would not require an external drug dissolution enhancing agent in the dissolution medium) and palm kernel oil-based 100 nm kinetically stable artemether lipid self-emulsifying system with rapid emulsification time. COVID-19 and Plasmodium falciparum-infected Africans with previous long exposure to malaria have manifested attenuated inflammatory cytokines more than malaria-naive patients. Therefore, the ingestion of artemether-lumefantrine with enhanced solubility may further promote blunting of cytokines. Therefore, this work was aimed at preparing (< 100 nm) stable artemether and aqueous acid-stable micro/large nano-scale (> 100 nm) lumefantrine lipid self-emulsifying system destined for improved antimalarial and anti-inflammatory activities.
Results
The droplet sizes of all the liquid artemether and lumefantrine formulations were between 8.95–39.88 and 1018–4195 nm, respectively. The loading efficiency for all the formulations was, between 72.91 ± 2.89 and 100.00 ± 0.29%. All the artemether and lumefantrine batches emulsified within the range of 3.90 ± 0.69 to 12.26 ± 0.69 s. Stable and transparent emulsions were formed on aqueous dilution to 1000 ml. The percentage drug released for artemether and lumefantrine ranged from 76.25 ± 2.98 to 99.22 ± 1.61%. The solid lipid self-emulsifying systems produced, had fair and passable flow properties. Differential scanning calorimetry revealed that the solid artemether and lumefantrine lipid self-emulsifying system were amorphous. Solidification with Neusilin FH2 or surfactant replacement with Kolliphor EL and Kollidon VA 64 fine prevented micro-or large nano-scale lumefantrine lipid self-emulsifying system from crystallisation in aqueous acid (pH 1.2). Higher antimalarial activity and remarkable anti-inflammatory effects (P < 0.05) favoured the lipid self-emulsifying formulations.
Conclusion
Optimal in vitro and in vivo results (enhanced antimalarial and anti-inflammatory activities) were obtained with kinetically stable lumefantrine micro/large nano-scale droplets and kinetically stable palm kernel oil-based (< 50 nm) artemether lipid self-emulsifying system droplets.
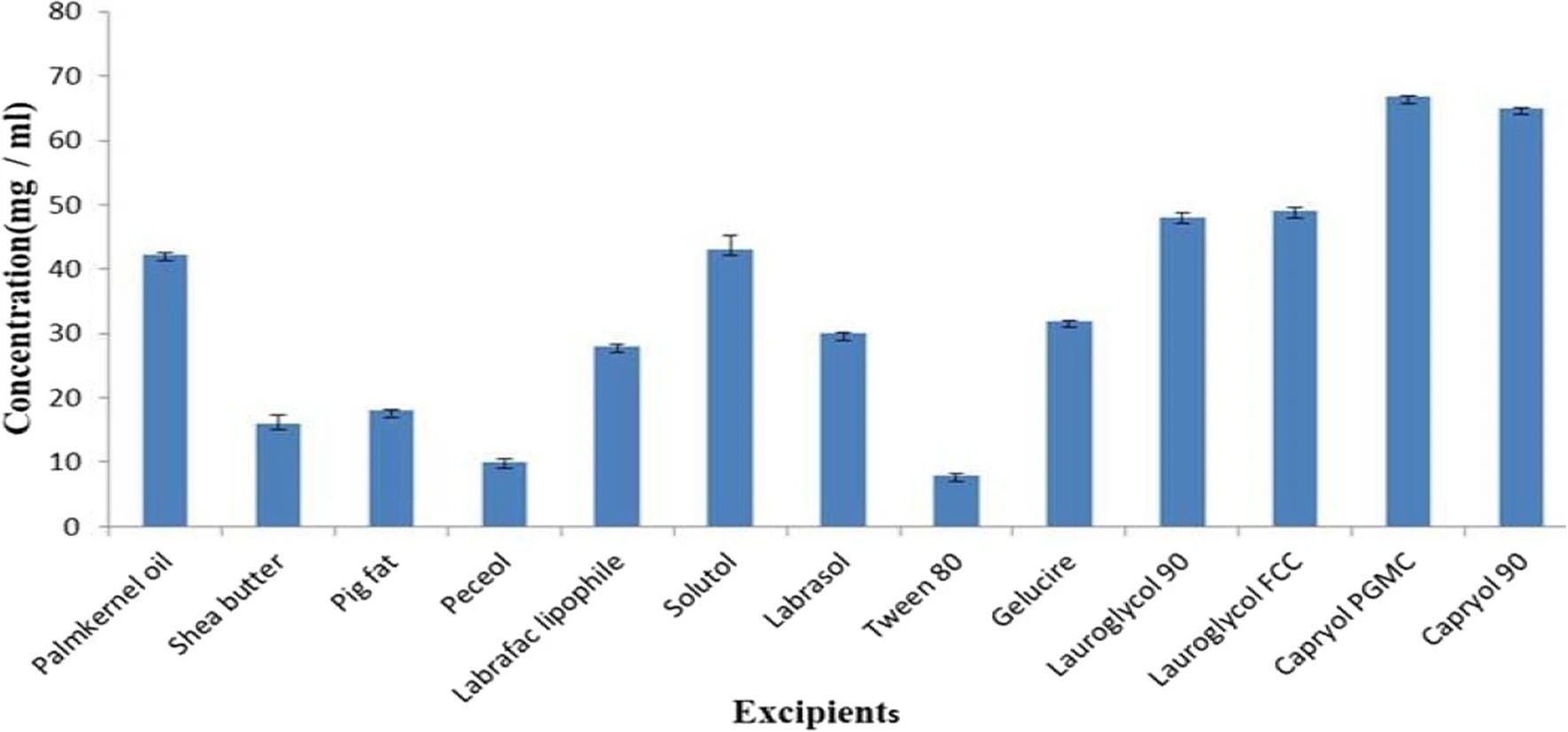
2.1 Materials
Shea butter was procured from Owode market Offa, Kwara– State Nigeria, Palm kernel oil (locally sourced from Ogige market, Nsukka, Nigeria), Oleic acid (Nasfco Scientific Supplies Ltd, London), Tween 80 (Sigma Aldrich, Germany), Kolliphor EL, Kolliphor RH 40, Kolliphor HS 15, Kollidon VA 64 (BASF, Germany), Labrasol, Gelucire, Lauroglycol 90, Lauroglycol FCC, Capryol 90, Capryol pgmc, Peceol, Labrafac lipophile (Gift samples from Gattefosse, France), Artemether (Hangzhou Dayang chemical, China), Lumefantrine (Hangzhou Dayang chemical, China), Neusilin FH2 (Fuji Chemical Industry Co. Japan), Hydrochloric acid (Sigma Aldrich, Germany), Absolute Methanol(Sigma Aldrich, Germany), Distilled water (STC Unit UNN). All other chemicals are of analytical grade and were used as such.
Download the full study as PDF here: Solubility enhancing lipid-based vehicles for artemether and lumefantrine destined for the possible treatment of induced malaria and inflammation: in vitro and in vivo evaluations
or read it here
Ugorji, O.L., Onyishi, I.V., Onwodi, J.N. et al. Solubility enhancing lipid-based vehicles for artemether and lumefantrine destined for the possible treatment of induced malaria and inflammation: in vitro and in vivo evaluations. Beni-Suef Univ J Basic Appl Sci 13, 3 (2024).
https://doi.org/10.1186/s43088-023-00446-w

