Supercritical antisolvent-fluidized bed for the preparation of dry powder inhaler for pulmonary delivery of nanomedicine
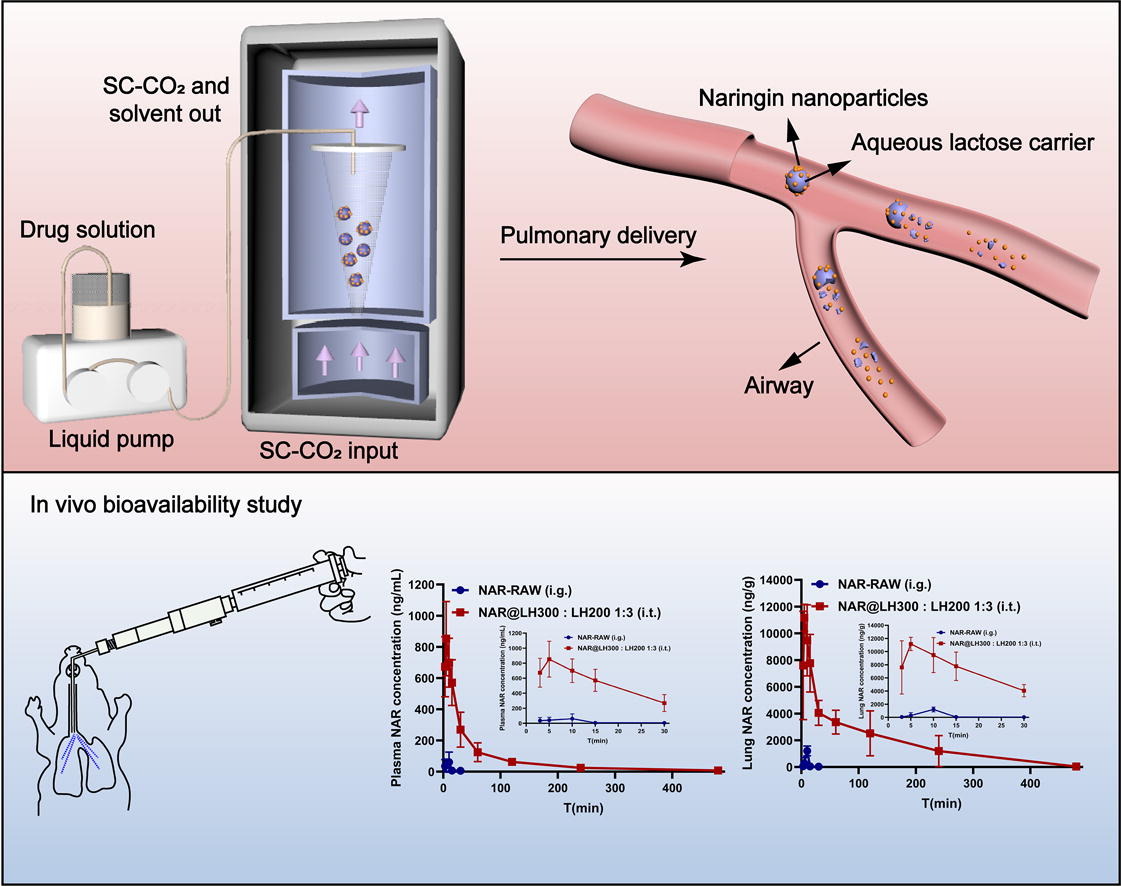
The supercritical antisolvent-fluidized bed coating process (SAS-FB) shows great potential as a technique to manufacture dry powder inhaler (DPI) that incorporate nanodrugs onto micronized matrix particles, capitalizing on the merits of both nanoparticle and pulmonary delivery. In this study, naringin (NAR), a pharmacologically active flavonoid with low solubility and in vivo degradation issues, was utilized as a model active pharmaceutical ingredient (API) to construct nanomedicine-based DPI through SAS-FB. It is showed that processed NAR exhibited a near-spherical shape and an amorphous structure with an average size of around 130 nm. Notably, SAS-FB products prepared with different fluidized matrices resulted in varying deposition patterns, particularly when mixed with a coarse lactose to enhance the fine particle fraction (FPF) of the formulations.
The FPF was positively associated with specific surface area of the SAS-FB products, while the specific surface area was directly related to surface roughness and particle size. In vitro dissolution studies using simulated lung fluid revealed that the NAR nanoparticles coated on the products were released immediately upon contact with solution, with a cumulative dissolution exceeding 90% within the first minute. Importantly, compared to oral raw NAR, the optimized DPI formulation demonstrated superior in vivo plasmatic and pulmonary AUC0→∞ by 51.33-fold and 104.07-fold respectively in a Sprague-Dawley rat model. Overall, SAS- FB technology provides a practical approach to produce nanomedicine DPI product that combine the benefits of nanoparticles with the aerodynamics properties of inhaled microparticles.
Read more here
Materials
No additional purification was conducted on the chemicals before their use. Professor Su Weiwei (Sun Yat-sen University, China) generously provided NAR (purity ≥ 98.0%) as a donation. Lactohale® 300 and Lactohale® 200 were kindly provided by DFE Pharma (Goch, Germany). Inhalac® 400 and Inhalac® 500 were a gift from Meggle Pharma (Wasserburg, Germany). Capsugel Co., Ltd. (Suzhou, China) provided the 3# Hydroxy propylmethyl cellulose (HPMC) capsule (Vcaps®).
Zhimin Ma, Xuejuan Zhang, Lu Ping, Zicheng Zhong, Xiubing Zhang, Xiaodong Zhuang, Guanlin Wang, Qiupin Guo, Shaofeng Zhan, Zhenwen Qiu, Ziyu Zhao, Qingguo Li, Dandong Luo, Supercritical antisolvent-fluidized bed for the preparation of dry powder inhaler for pulmonary delivery of nanomedicine, International Journal of Pharmaceutics,
2023, 123580, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123580.
Read also the other article on Dry Powder Inhalers (DPI):
- Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate
- Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery
- Evaluation of the Effects of Storage Conditions on Spray-Dried siRNA-LNPs Before and after Subsequent Drying


