Fabrication of TPGS decorated Etravirine loaded lipid nanocarriers as a neoteric oral bioavailability enhancer for lymphatic targeting
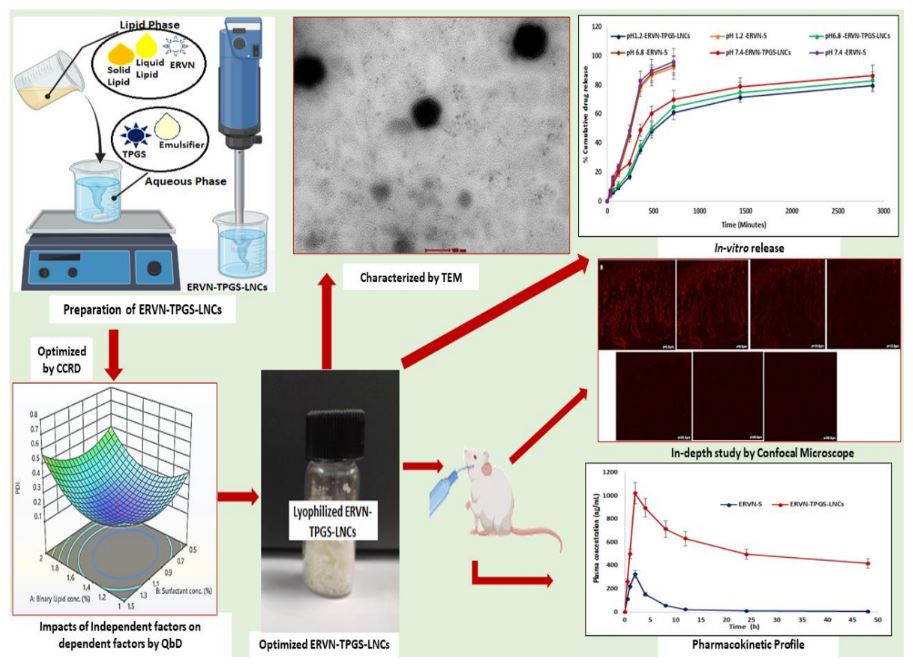
Etravirine (ERVN) is a potential NNRTI (non-nucleoside reverse transcriptase inhibitor) in treating HIV infection. It possesses extremely low oral bioavailability. The present research aims to optimize the formulation and characterization of TPGS-enriched ERVN-loaded lipid-based nanocarriers (LNCs) for HIV-infected patients. The formulation, ERVN-TPGS-LNCs, was optimized by CCRD using a modified-solvent evaporation process. Various characterization parameters of LNCs were evaluated, including globule size of 121.56 ± 2.174 nm, PDI of 0.172 ± 0.042, the zeta potential of -7.32 ± 0.021 mV, %EE of 94.42 ± 8.65% of ETR and %DL was 8.94 ± 0.759% of ERVN and spherical shape was revealed by TEM. PXRD was also performed to identify the crystallinity of the sample. In-vitro drug release showed % a cumulative drug release of 79.77 ± 8.35% at pH 1.2 and 83.23 ± 9.11% at pH 6.8, respectively, at the end of 48h compared to pure drug suspension (ERVN-S). Further, the intestinal permeation study and confocal microscope showed approximately ~3-fold and ~2-fold increased permeation in ERVN-TPGS LNCs and ERVN-LNCs across the gut sac compared to ERVN-S. Hemolysis compatibility and lipolysis studies were performed to predict the in-vivo fate of the formulation. The pharmacokinetic study revealed a 3.13-fold increment in the relative bioavailability, which agrees with the ex-vivo studies, and lymphatic uptake was validated by using cycloheximide (CYHD) along with designed formulation, which leads to lowering AUC of ERVN-TPGS-LNCs. Thus, this study ensures that ERVN-TPGS-LNCs take lymphatic uptake to minimize the first-pass metabolism followed by improved oral bioavailability of EVN. Thus, the enhanced bioavailability of ERVN can reduce the high dose of ERVN to minimize the adverse effects related to dose-related burden.
2.1.Materials
Etravirine was gifted by Hetero Pharmaceutical, Hyderabad, India. Vitamin E TPGS was purchased from Sigma Aldrich, India. BASF provided Labrafil M-2125 CS, Peceol, Labrafil M 1944CS, Maisine-300, Castrol oil, Plurol Oleique, Canola oil, Sesame oil, Labrasol, Capmul PG 12, Miglycol-829, Lauroglycol FCC, Captex-350, Miglycol-840, Captex-300, Neobee M-20, Labrafac WL-1349, Oleic acid, Lauroglycol-90, Capmul MCM-28, Caprol PGE-860, Precirol ATO 5, Compritol 888 ATO, Gelucire 44/14, Gelot 64, Stearic acid, Glyceryl monostearate, Apifil, Gelucire 50/13, Cremophor EL, Tween 80, Cremophore RH 40, Solutol H-15, Tween 20, and Poloxamer 407, Poloxamer 188. HPLC-grade water, methanol, and acetonitrile were purchased from Sigma Aldrich, India. Deionized water was procured from Millipore Instruments, MA, USA, available in CIF, Jamia Hamdard, New Delhi.
Download the full study PREPRINT as PDF here: Fabrication of TPGS decorated Etravirine loaded lipid nanocarriers as a neoteric oral bioavailability enhancer for lymphatic targeting
or read it here
Abdul Muheem, Mohd. Wasim, Eman Aldosari et al. Fabrication of TPGS decorated Etravirine loaded lipid nanocarriers as a neoteric oral bioavailability enhancer for lymphatic targeting, 26 September 2023, PREPRINT (Version 1) available at Research Square, https://doi.org/10.21203/rs.3.rs-3342708/v1
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


