A review on co-processed excipients used in direct compression of tablet dosage form
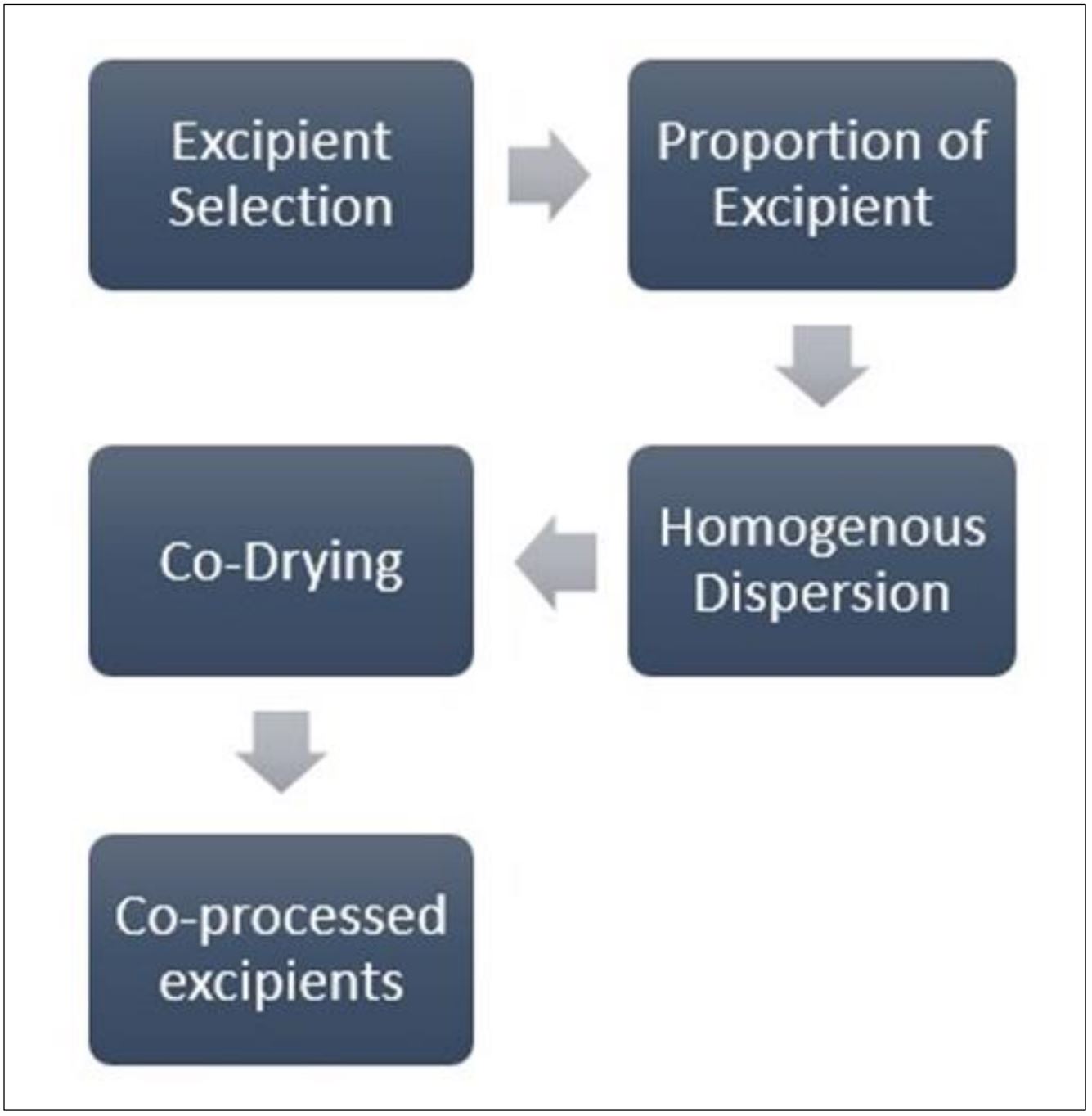
Co-processed excipients may enhance functionality and reduce drawbacks of traditional excipients for the manufacture of tablets on a commercial scale. The following study aimed to characterize a range of co-processed excipients that may prove suitable for dispersible tablet formulations prepared by direct compression. The dosage form that is used the most is tablets. Their accessibility, simplicity of administration, consistency, and affordability are advantages. Direct compression is the most straightforward method for making tablets, despite the fact that it comes with several challenges, including those connected to the homogeneity and mass variation of the content, disintegration, dissolution, and the radial hardness of the tablets.
In today’s world, “co-processed excipients,” which include frequently processed mixtures of fillers, binders, disintegrants, lubricants, and other excipients, are becoming more popular. Spray drying, fluid bed granulation, wet granulation, melt granulation, dry granulation, and co-crystallization are used to create these mixes. This review article lists technologies, co-processed excipients that are commercially available, and excipients that are typically utilized to make them.
| Co-processed excipients | Trade name | Manufacturer | Advantages |
|---|---|---|---|
| Mannitol, hydroxypropyl methylcellulose | Pearlitol | Roquette | Used in direct compression of controlled oral tablets, binder |
| Microcrystalline cellulose, colloidal silicon dioxide | Dicom Sanaq® SP206 | Pharmatrans Sanaq | Used in direct compression of hygroscopic and moisture sensitive apis and has excellent flow properties |
| Lactose monohydrate, cellulose micro crystalline | Dicom Sanaq ML 011 | Pharmatrans Sanaq | Enhance the stability and effectiveness during manufacturing product process and high compressibility, superior dilution properties and rapid disintegrating |
| Spray granulated d-mannitol and croscarmellose sodium | Parteck® ODT | Merck | Directly compressible excipient designed for orally disintegrating tablets and provided rapid disintegration and exceptional strength as well as a pleasant taste and mouthfeel |
| Hypromellose and lactose | Retalac® | Meggle | Improved flow and bendability, enhancing compatibility in direct compression |
| 70 % alpha-lactose monohydrate, 20 % microcrystalline cellulose (mcc) and 10 % white, native corn starch | Combilac® | Meggle | Improved compaction properties compared to an equivalent admixture of individual ingredients, providing robust tablets with minimal friability, and assuring rapid, hardness independent tablet disintegration for effective api release |
| Microcrystalline cellulose, colloidal silicon dioxide, mannitol, fructose, crospovidone | Prosolv® ODT G2 | JRS Pharma | Smooth and creamy mouthfeel, Excellent flowability, excellent blending characteristics for improved content uniformity, High patient compliance |
| Crospovidone, dextrose monohydrate, mannitol, monohydrate lactose | Disintequik™ ODT | Kerry | Direct tableting operations for use in orally disintegrating tablets. It can be blended with a flavor, active, suitable lubricant, additional sweetener if desired |
| Microcrystalline cellulose, monohydrate lactose | Disintequik MCC | Kerry | Direct tableting operations where hard tablets and fast disintegration are required. |
| Carmellose, crospovidon, mannitol, microcrystalline cellulose | Granfiller-D | Daicel Group and Nichirin-Chem | Direct compression odts, high tablet hardness and rapid disintegration |
| Calcium carbonate & polyvinyl pyrrolidone | Dicom-DC® S-604 | Gangwal Healthcare Private Limited | Excellent flowability, better compressibility & uniform particle size distribution. |
| Sucrose 3%, dextrin microcrystalline Cellulose, silicon dioxide | Dipa prosolv | Penwest Pharmaceuticals Company | Directly compressible, better flow, reduced sensitivity to Wet granulation, better hardness of tablet, reduced friability |
| Lactose and cellulose | Cellactose | Meggle | High compressibility, good mouth feels, better tableting at low cost |
| Sucrose and dextrin | Dipac | Penwest pharm | Directly compressible grade |
| Microcrystalline cellulose and silicon dioxide | Prosolv | Penwest pharmaceuticals | Better flow, reduced sensitivity to wet granulation, better hardness of tablet, reduced friability |
| Microcrystalline cellulose and guar gum | Avicel CE-15 | FMC Corp. | Less grittiness and minimal chalkiness |
| Calcium carbonate and sorbitol | Formaxx | Merck | High compressibility, excellent taste masking, free flow, superior content uniformity, controlled particle size distribution |
| Microcrystalline cellulose and lactose | Microcelac | Meggle | Capable of formulating high dose, small tablets with Poorly flowable active ingredients |
| Lactose and maize starch | Starlac | Meggle | Good flowability due to spray drying, the acceptable crushing force due to lactose content and rapid disintegration |
| Lactose, 3.2 % Kallidone Kallidone CL | Ludipress | BASF, Ludwigshafen, Germany | Low degree of hygroscopicity, good flowability, tablet hardness independent of machine speed |
| Lactose, 25 % cellulose | Cellactose | Meggle | Highly compressible, good Mouthfeel, better tableting at low cost |
Download the full article as PDF here: A review on co-processed excipients used in direct compression of tablet dosage form
or read it here
Paka Bhavana and M Sunitha Reddy, A review on co-processed excipients used in direct compression of tablet dosage form, Department of Pharmaceutics, Centre for Pharmaceutical Sciences, JNTUH UCEST, JNTUH, Hyderabad, Telangana-500085, India. GSC Biological and Pharmaceutical Sciences, 2023, 23(01), 212–219
Publication history: Received on 06 January 2023; revised on 17 April 2023; accepted on 20 April 2023, Article DOI: https://doi.org/10.30574/gscbps.2023.23.1.0100

