SEDEX—Self-Emulsifying Delivery Via Hot Melt Extrusion: A Continuous Pilot-Scale Feasibility Study
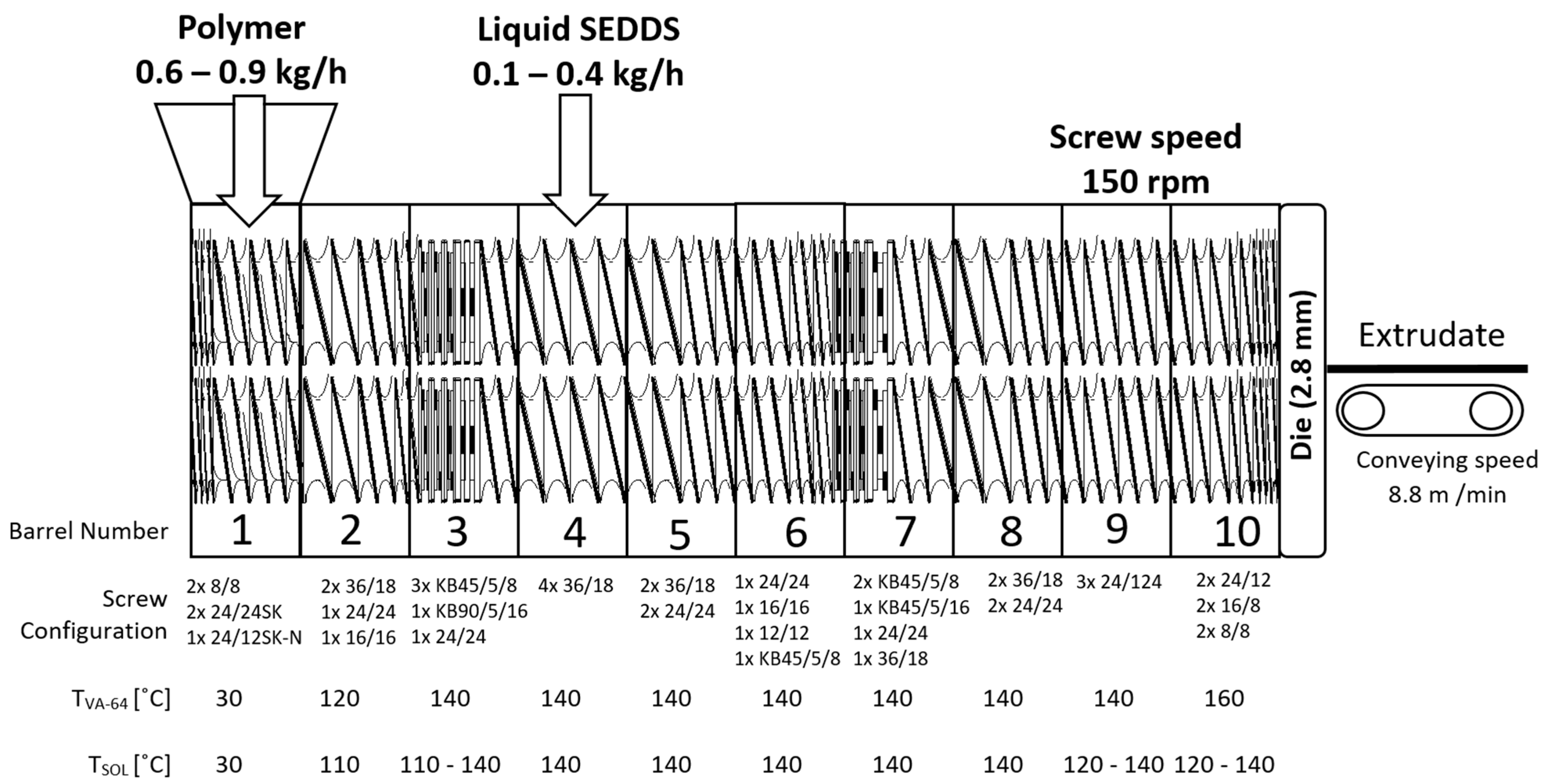
The aim of this study was to develop a continuous pilot-scale solidification and characterization of self-emulsifying drug delivery systems (SEDDSs) via hot melt extrusion (HME) using Soluplus® and Kollidon® VA-64. First, an oil-binding capacity study was performed to estimate the maximal amount of SEDDSs that the polymers could bind. Then, HME was conducted using a Coperion 18 mm ZSK18 pilot plant-scale extruder with split-feeding of polymer and SEDDS in 10, 20, and 30% w/w SEDDSs was conducted. The prepared extrudates were characterized depending on appearance, differential scanning calorimetry, wide-angle X-ray scattering, emulsification time, droplet size, polydispersity index, and cloud point.
The oil-binding studies showed that the polymers were able to bind up to 50% w/w of liquid SEDDSs. The polymers were processed via HME in a temperature range between 110 and 160 °C, where a plasticizing effect of the SEDDSs was observed. The extrudates were found to be stable in the amorphous state and self-emulsified in demineralized water at 37 °C with mean droplet sizes between 50 and 300 nm. A cloud point and phase inversion were evident in the Soluplus® samples. In conclusion, processing SEDDSs with HME could be considered a promising alternative to the established solidification techniques as well as classic amorphous solid dispersions for drug delivery.
Download the full article as PDF here SEDEX—Self-Emulsifying Delivery Via Hot Melt Extrusion_A Continuous Pilot-Scale Feasibility Study
or read it here
Materials
Kollidon® VA-64 (copovidone, VA64), Soluplus® (Poly(vinyl caprolactam-covinylacetate-ethylene glycol) graft polymer, SOL), and Kolliphor® RH40 (PEG-40 Hydrogenated Castor Oil) were received from BASF SE (Ludwigshafen am Rhein, Germany). Plasdone® S-630 (PVPVA64, PL_630) and Plasdone® S-630 Ultra (PVPVA64, PL_630U) were delivered from Ashland Inc. (Düsseldorf, Germany). Capmul® MCM EP (Glycerol Monocaprylocaprate Type I EP) was provided by Abitec Corp (Janesville, WI, USA). Transcutol® (diethylene glycol monoethyl ether) and Labrafac® lipophile WL 1349 (medium chain triglycerides) were received from Gattefossé (Saint-Priest, France).
Zupančič, O.; Doğan, A.; Matić, J.; Kushwah, V.; Alva, C.; Spoerk, M.; Paudel, A. SEDEX—Self-Emulsifying Delivery Via Hot Melt Extrusion: A Continuous Pilot-Scale Feasibility Study. Pharmaceutics 2022, 14, 2617. https://doi.org/10.3390/pharmaceutics14122617

