Microcrystalline cellulose promotes superior direct compressed Boesenbergia rotunda (L.) Mansf. extract tablet properties to spray-dried rice starch and spray-dried lactose
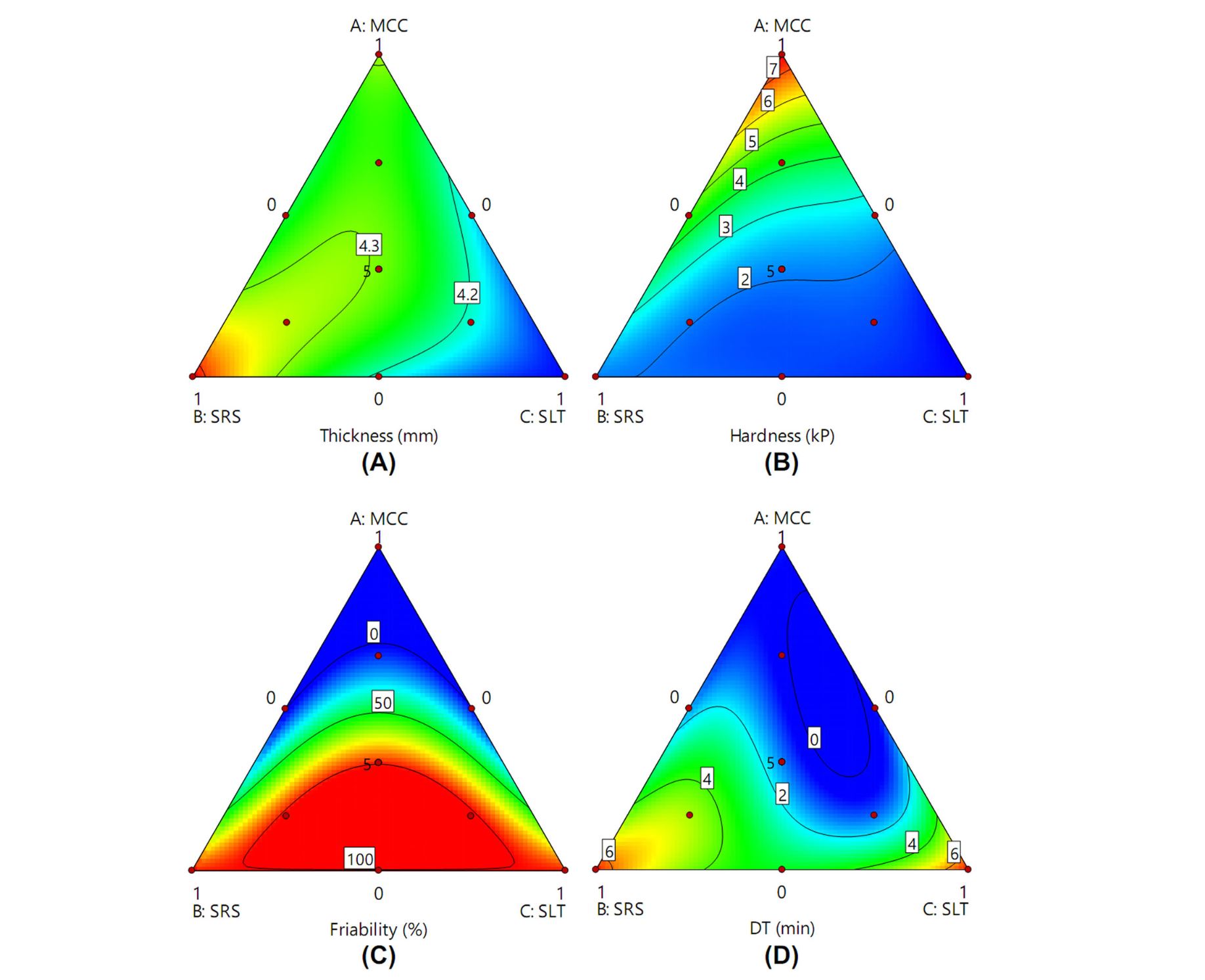
This work aimed to select tablet diluent of Boesenbergia rotunda (L.) Mansf. extract tablet. Three tablet diluents, including microcrystalline cellulose (MCC), spray-dried rice starch, and spray-dried lactose were used. MCC exhibited superior performance to the other diluents by providing the hardest tablet, the lowest friability, and the shortest disintegration time. The Box-Behnken design was used to evaluate the effect of hydrophobic excipients when MCC was used as diluent. The optimal formulation was composed of fumed silica 1%, magnesium stearate 1%, and talcum 2%. The tablets had suitable hardness, low friability, and short disintegration time. The marker pinocembrin could be dissolved by 82% within 4 h. Although the marker decreased after three months of stability testing, the antioxidant activity of the formulation remained. In conclusion, MCC was shown to be superior to the other diluents, and the optimal formulation could be used to prepare Boesenbergia rotunda (L.) Mansf. extract tablet as a food supplement.
Introduction
The size of the antioxidants market will surpass approximately 9,000 million USD by 2026 at a 5.3% compound annual growth rate over the assessment period between 2020 and 2026 (GlobeNewswire, 2021). A natural antioxidant is an interesting source of compounds. Boesenbergia rotunda (L.) Mansf. is one of several natural antioxidants that have been marketed recently. B. rotunda or fingerroot is a plant that belongs to the Zingiberaceae family. It is used as a food ingredient and herbal medicine in Southeast Asia as well as Indo-China. It possesses several biological and pharmacological activities, i.e. anti-inflammatory effect (Isa et al., 2012), antimicrobial activity (Bhamarapravati, Juthapruth, Mahachai, & Mahady, 2006; Jitvaropas et al., 2012), antioxidant activity (Isa et al., 2012; Jitvaropas et al., 2012), aphrodisiac activity (Ongwisespaiboon & Jiraungkoorskul, 2017), cytotoxic effect (Isa et al., 2012), vasorelaxant effect (Adhikari et al., 2020), wound healing (Jitvaropas et al., 2012; Ruttanapattanakul et al., 2021), treatment of functional dyspepsia (Chitapanarux, Lertprasertsuke, & Toworakul, 2021), etc. During the Covid-19 pandemic, herbal plants were screened for anti-SARS-CoV-2 activity.
Among them, B. rotunda extract and its isolated compound panduratin A exhibited anti-SARS-CoV-2 activity (Kanjanasirirat et al., 2020). Recently, several fingerroot extract products have been launched in the market. Several products with various doses of B. rotunda extract have been registered. The 200 mg B. rotunda extract per tablet was selected in this work based on the dose of marketed products that have been sold in Thailand (Thai Food & Drug Administration, 2022). However, there is no regulation concerning the standard dose of B. rotunda extract used for food supplements in Thailand (Thai Food & Drug Administration, 2017). In Thailand, numerous products of B. rotunda powder as well as its extract are typically prepared in capsule dosage form, due to it being easily operated in small herbal factories or small to medium-sized enterprises. Numerous publications have reported on the biological and pharmacological activities of B. rotunda extract. However, there are limited numbers of publications based on the formulation development of B. rotunda powder or its extract. An innovative product incorporating B. rotunda extract with a hydrogel wound dressing has also been reported (Eakwaropas et al., 2019).
Recently, tablets have become the most popular form of solid oral dosage. Direct compression is the first choice for the preparation of tablets. Direct compression exhibits several advantages compared to wet granulation or dry granulation methods, such as saving equipment, energy, space, and time. It also reduces the risk of cross-contamination due to fewer operating procedures, reducing the risk of microbial growth and the risk of degradation of moisture as well as thermal-sensitive drugs due to no water being used. Generally, tablets prepared by direct compression exhibit a faster dissolution rate. However, it requires desired pharmaceutical excipient properties such as good flowability, compressibility, and compactability (Mura, Valleri, Baldanzi, & Mennini, 2019).
The direct compression diluents included in this work were microcrystalline cellulose (MCC), spray-dried rice starch (SRS), and spray-dried lactose (SLT). MCC is one of the most popular cellulose derivatives used as tablet diluent. It exhibits good compressibility, so it is usually included in directly compressed tablet formulations (Zhao, Zhao, Lin, & Shen, 2022). It also exhibits disintegration properties (Rowe, Sheskey, & Quinn, 2009). However, it also displays poor flow properties (Jivraj, Martini, & Thomson, 2000). Its angle of repose, bulk density, and tapped density was 28–29°, 0.34 g/cm3. and 0.48 g/cm3, respectively (Rowe et al., 2009). SRS is one of the direct compression fillers. It is an insoluble, neutral, and free-flowing powder (Vongsurakrai & Varavinit, 2010). It also exhibits disintegration properties with a poor flow similar to MCC (Jivraj et al., 2000). Its angle of repose, bulk density, and compressibility were 35–52°, 0.37–0.45 g/cm3, and 20–36%, respectively (Bergthaller, Varavinit, & Wongsagonsup, 2005).
Developed SRS (Era-Tab®) had excellent flowability comparable to dibasic calcium phosphate but superior to MCC, lactose, and pregelatinized starch. However, MCC was superior to other diluents in terms of dilution potential or carrying capacity (Mitrevej, Sinchaipanid, & Faroongsarng, 1996). SLT is usually used as a tablet binder, filler-binder, and flow aid in direct compression tableting. It is a free-flowing powder that disintegrates by dissolution. However, it requires high compressional force to produce hard tablets when compared with MCC (Jivraj et al., 2000). Its angle of repose, bulk density, and tapped density were 28–29°, 0.57–0.67 g/cm3, and 0.67–0.78 g/cm3, respectively (Rowe et al., 2009). The selection of direct compression diluents may be an important step to obtain the desired direct compressed tablet properties.
Hydrophobic excipients (e.g. fumed silica, magnesium stearate, and talcum) are also included in tablet formulations to promote desired tablet properties. Lubricants are used to prevent the sticking of the tablet to the punch faces as well as to reduce friction between the tablets and the die wall during the compression and ejection steps. Magnesium stearate is the most common lubricant used in tablet formulation. Other lubricants such as stearic acid and calcium stearate are rarely used. Glidants are used to improve the flowability of the powder blend. Several compounds can be used as glidants such as fumed silica, starch, and talcum. Anti-adherents prevent the tablet from sticking to the die wall and punch faces. Talcum, starch, and magnesium stearate can be used as anti-adherents in tablet formulation (Chowhan, 2020). However, using excessive hydrophobic excipients retarded drug dissolution from a solid dosage form (Rowe et al., 2009). Thus, optimization of hydrophobic excipients is important to obtain suitable tablet properties.
This work aimed to select a tablet diluent of direct compressed B. rotunda extract tablet. Three tablet diluents including MCC, SRS, and SLT were included in the simplex lattice design. The most suitable diluent will be used to prepare B. rotunda extract tablets by varying hydrophobic excipients including, fumed silica, magnesium stearate, and talcum using the Box-Behnken design. Furthermore, the stability of B. rotunda extract tablets was also evaluated. The authors expected that the optimal B. rotunda extract tablet formulation could be used to prepare antioxidant food supplement products.
Download the full article as PDF here Microcrystalline cellulose promotes superior direct compressed Boesenbergia rotunda (L.) Mansf. extract tablet properties to spray-dried rice starch and spray-dried lactose
or read it here
Materials
Standard pinocembrin was purchased from Chengdu Biopurify Phytochemicals Ltd., Sichuan, China. Fingerroot extract (yellow powder; moisture content of 3.1%; bulk density of 0.45 g/cm3; flavonoids content of 267.2 mg%) was purchased from Specialty Natural Product Co., Ltd., Chonburi, Thailand. MCC (Comprecel® M102) was purchased from Maxway Co., Ltd., Bangkok, Thailand. SRS (Era-Tab®) was purchased from Erawan Pharmaceutical Research and Laboratory Co., Ltd., Bangkok, Thailand. SLT (FlowLac®) was purchased from Molkerei Meggle Wasserburg GmbH & Co., Wasserburg am Inn, Germany. Magnesium stearate was purchased from Changzhou Kaide Imp. & Exp. Co., Ltd., Changzhou, China. Talcum was purchased from Nitika Pharmaceutical Specialities Pvt. Ltd., Nagpur, India. Fumed silica was purchased from P.C. Drug Center, Bangkok, Thailand. Sodium lauryl sulphate (SLS) was purchased from EMD Millipore Corporation, Massachusetts, USA. 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Pte Ltd., Ascent, Singapore. Acetonitrile (HPLC grade) was purchased from Fisher Chemical, Leicestershire, UK.
Jirapornchai Suksaereea, Chaowalit Montonb, Natawat Chankanacand LaksanaCharoencha, Microcrystalline cellulose promotes superior direct compressedBoesenbergia rotunda(L.) Mansf. extract tablet properties to spray-driedrice starch and spray-dried lactose, ARAB JOURNAL OF BASIC AND APPLIED SCIENCES2023, VOL. 30, NO. 1, 13–25https://doi.org/10.1080/25765299.2022.2153527
Read more on Magnesium Stearate as a pharmaceutical excipient here:


