Evaluation of the Formulation Parameter-Dependent Redispersibility of API Nanoparticles from Fluid Bed Granules
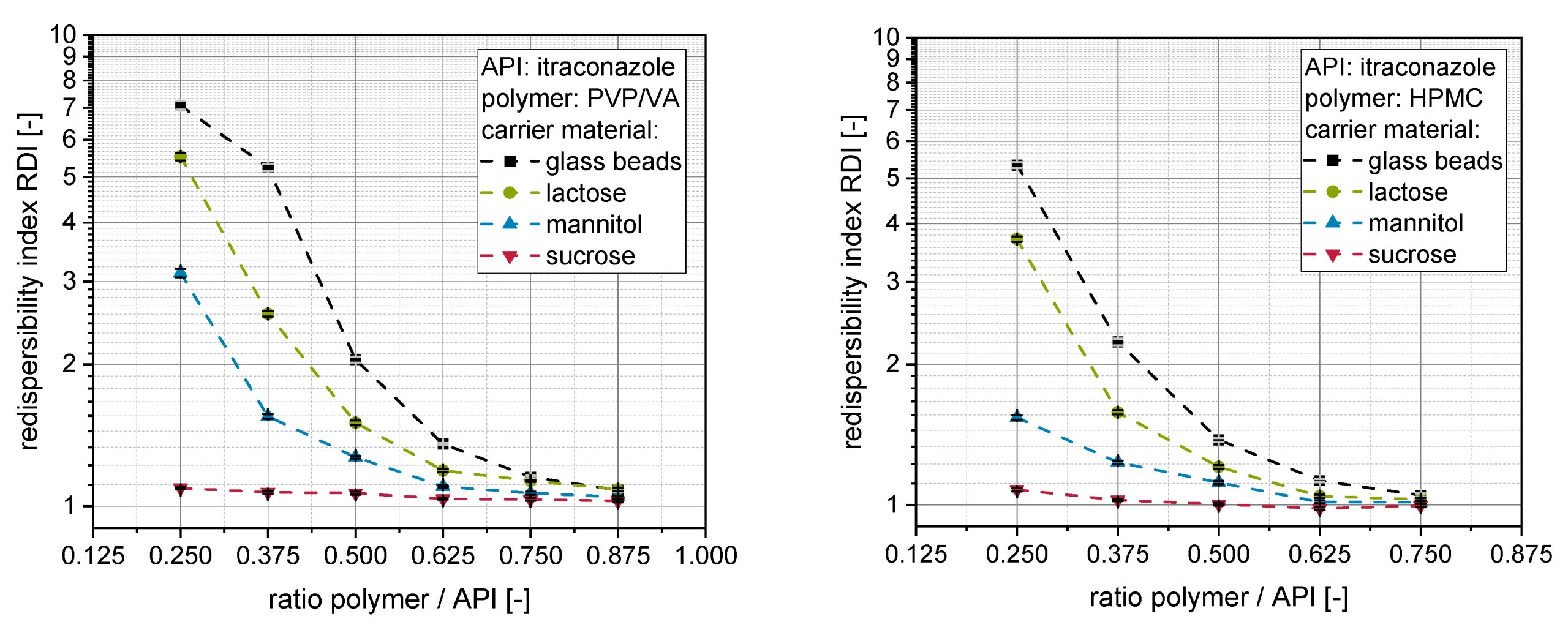
The production of nanosuspensions of poorly soluble active pharmaceutical ingredients (API) is a popular technique to counteract challenges regarding bioavailability of such active substances. A subsequent drying of the nanosuspensions is advantageous to improve the long-term stability and the further processing into solid oral dosage forms. However, associated drying operations are critical, especially with regard to nanoparticle growth, loss in redispersibility and associated compromised bioavailability. This work extends a previous study regarding the applicability of an API (itraconazole) nanosuspension as a granulation liquid in a fluidized bed process with focus on the influence of applied formulation parameters on the structure of obtained nanoparticle-loaded granules and their nanoparticle redispersibility. Generally, a higher dissolution rate of the carrier material (glass beads, lactose, mannitol or sucrose) and a higher content of a matrix former/hydrophilic polymer (PVP/VA or HPMC) in the granulation liquid resulted in the formation of coarser and more porous granules with improved nanoparticle redispersibility. HPMC was found to have advantages as a polymer compared with PVP/VA. In general, a better redispersibility of the nanoparticles from the granules could be associated with better dispersion of the API nanoparticles at the surface of the granules as deduced from the thickness of nanoparticle-loaded layers around the granules. The layer thickness on granules was assessed by means of confocal Raman microscopy. Finally, the dispersion of the nanoparticles in the granule layers was exemplarily described by calculation of theoretical mean nanoparticle distances in the granule layers and was correlated with data obtained from redispersibility studies.
Conclusion
The findings of the present study highlight the effects of formulation changes on the granules obtained from fluidized bed granulation using an itraconazole nanosuspension as granulation liquid. Variation of formulation parameters regarding the type of hydrophilic polymer (PVP/VA and HPMC), the ratio of polymer to API and the starting carrier material resulted in significant differences in redispersibility of nanoparticle-loaded granules. In this regard, the findings of a previous study [20] concerning the influence of the carrier material on naproxen-containing granules, as well as the applied type and amount of Pharmaceutics 2022, 14, 1688 15 of 17 additional polymer in a naproxen nanosuspension, could be confirmed for an itraconazole nanosuspension. Additionally, the observations were extended by applying insoluble glass beads as a model carrier material. Corresponding data align with data obtained for soluble carrier materials (i.e., lactose, mannitol and sucrose), illustrating the importance of the carrier particle surface dissolution and subsequent resolidification. For soluble carrier materials, a high intrinsic dissolution rate of the carrier material facilitates the preservation of nanoparticle size and dispersity within the nanoparticle-loaded granules.
Download the full article as PDF here Evaluation of the Formulation Parameter-Dependent Redispersibility of API Nanoparticles from Fluid Bed Granules
or read it here
Materials
In the present study, micronized itraconazole (kindly provided by Novartis Pharma AG, Basel, Switzerland) was used as a poorly water-soluble model API. During milling, either a vinylpyrrolidone–vinyl acetate copolymer (PVP/VA, Kollidon VA 64, gift from BASF SE, Ludwigshafen am Rhein, Germany) or hydroxypropyl methyl cellulose (HPMC, Pharmacoat 603, gift from Shin-Etsu Chemical, Chiyoda, Japan) was used as a steric stabilizer in combination with sodium dodecyl sulfate (SDS, Sigma Aldrich, St. Lois, MO, USA). The same polymers PVP/VA and HPMC were also used as additional polymeric excipients during fluidized bed granulation.
Sucrose (Nordzucker AG, Braunschweig, Germany), mannitol (Pearlitol 160 C, Roquette Frères, Lestrem, France) and lactose monohydrate (lactose, Granulac 140, Meggle AG, Wasserburg am Inn, Germany) were used as soluble carrier materials during granulation trials. As the applied sucrose showed a significantly different mean particle size in comparison to mannitol and lactose, fines of sucrose were separated by sieving in order to harmonize the particle size of all investigated carrier materials. Sieving was carried out in batches of 100 g sucrose using an air jet sieve (e200 LS, Hosokawa Alpine AG, Augsburg, Germany) equipped with a 20 µm sieve for 15 min at a pressure of 4000 Pa. In order to remove the fines sufficiently, the procedure was repeated twice. The separated fines smaller than 20 µm were discharged, and sieve retention was applied as carrier material during granulation trials. For particle size distribution of soluble carrier materials, see Figure 1. Additionally, glass beads (SiLibeads SOLID 40–70 µm, Sigmund Lindner GmbH, Warmensteinach, Germany) were applied as insoluble model carrier material during the granulation process.
Wewers, M.; Finke, J.H.; Czyz, S.; Van Eerdenbrugh, B.; John, E.; Büch, G.; Juhnke, M.; Bunjes, H.; Kwade, A. Evaluation of the Formulation Parameter-Dependent Redispersibility of API Nanoparticles from Fluid Bed Granules. Pharmaceutics 2022, 14, 1688. https://doi.org/10.3390/pharmaceutics14081688

