Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies
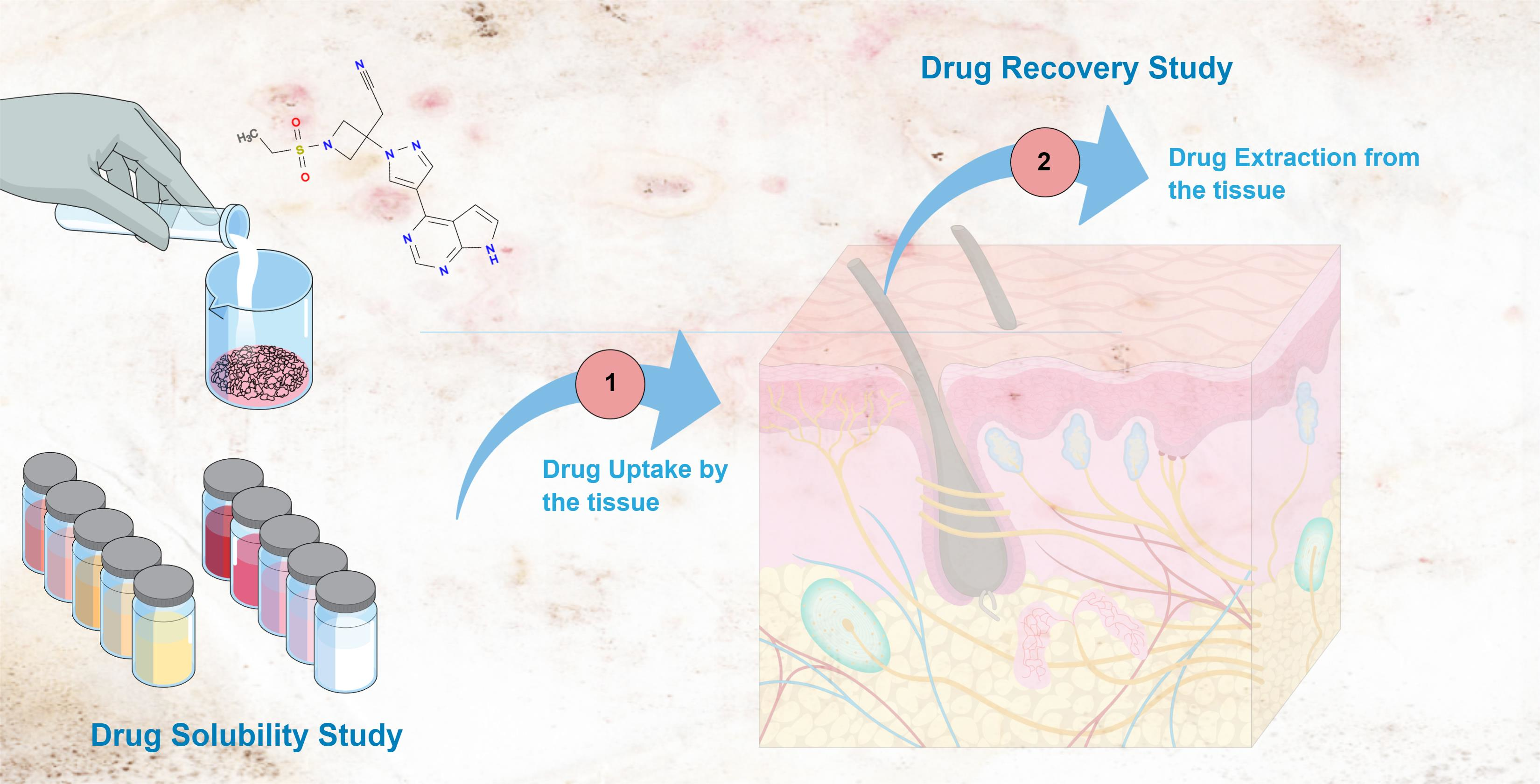
The low water solubility of baricitinib (BCT) limits the development of new formulations for the topical delivery of the drug. The aims of this study were to assess the solubility of BCT in different solvents, including Transcutol, a biocompatible permeation enhancer that is miscible in water, to evaluate the drug uptake in human skin and porcine tissues (sclera, cornea, oral, sublingual, and vaginal), and to subsequently extract the drug from the tissues so as to determine the drug recovery using in vitro techniques. Analytical methods were developed and validated for the quantification of BCT in Transcutol using absorption and fluorescence spectroscopies, which are complementary to each other and permit the detection of the drug across a broad range of concentrations. Results show that Transcutol permits an increased drug solubility, and that BCT is able to penetrate the tissues studied. The solutions of BCT in Transcutol were stable for at least one week. Hence, Transcutol may be a suitable solvent for further development of topical formulations.
Introduction
Topical delivery is a noninvasive route and an alternative to oral administration. The topical route involves the skin and the nasal, buccal, sublingual, ophthalmic, rectal, and vaginal mucosae. Some patients struggle with oral administration, while in contrast the topical route is easy to administer, which may improve the patients’ compliance; some medications administered orally cause digestive side-effects, but the topical route may avoid this inconvenience. Additionally, drug abuse through the topical dosage form is lower than under oral administration. Topical delivery seeks the permeation of drugs through the skin or mucosae [1]. However, drugs face some barriers in penetrating into the tissues, these can include the presence of mucus on the mucosae, low water content, or the existence of the stratum corneum, which is the outermost skin layer and has the main barrier function [2].
Baricitinib (BCT), named 2-[1-ethylsulfonyl-3-[4-(7 H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile according to IUPAC (Figure 1), is an oral selective and reversible Janus-associated kinase (JAK) inhibitor, which modulates the signaling pathway. It has a known anti-inflammatory profile in patients with autoimmune diseases. The BCT mechanism of action consists of inhibiting the signal transduction of IL-6, IL-12, IL-20, IL-22, IL-23, and IFN-γ [3,4,5,6]. BCT was approved by the European Medicines Agency (EMA) for the treatment of moderately to severely active rheumatoid arthritis (RA) in adults and for the management of specific cases of atopic dermatitis [7]. Recently, the US Food and Drug Administration (FDA) approved BCT for emergency use in the treatment of COVID-19 due to its capability of modulating the immunopathology associated with SARS-CoV-2 infection, as well as for the treatment of alopecia areata in adults [8,9].
The therapeutic effectiveness and safety of BCT have been investigated, showing sufficient effectiveness and tolerability in clinical trials [10,11]. It is typically administered orally, after which 80% oral bioavailability has been reported in healthy human subjects, but it decreased by 11–18% in the presence of high-fat meals [8]. BCT has a relatively low molecular weight (371.42 Da), and, although BCT is very poorly soluble in water (0.357 mg/mL to 0.46 mg/mL at 25 °C) [3,12], it is bound to plasma and serum proteins; thus, its oral administration implies systemic distribution. After intravenous administration, its volume of distribution is high (76 L), confirming that distribution to the tissues is significant, which is also in line with its low water solubility.
The typical oral doses range from 1 to 4 mg, indicating that BCT is a very potent drug, especially when its high distribution volume is considered [13]. Therefore, oral administration requires sufficiently high doses so as to achieve therapeutic concentrations in the tissues undergoing inflammatory processes. However, at the same time, it implies potential secondary effects. The achievement of efficacy while decreasing potential secondary effects can be achieved by topical administration, using administration routes such as dermal, ophthalmic, injectable, etc. [6,14,15].
Formulation approaches for the enhancement of the bioavailability of BCT are very scarce in the literature [16,17], most probably due to its very low solubility in water. Moreover, BCT is poorly soluble in ethanol (0.40 mg/mL), but it is freely soluble in organic solvents such as dimethyl sulfoxide (74 to 165.1 mg/mL) and dimethylformamide (50 mg/mL) [12,16]. The toxicity of the organic solvents might have also influenced in the lack of suitable formulations for alternative routes of administration. In contrast, BCT is soluble in PEG-400 (72.4 mg/mL) [12], which is in turn soluble in water. However, PEG-400 is a polymer of nine units of ethylene glycol, and, although it is considered biocompatible, it has been reported that increasing the number of ethylene glycol units of the PEG decreases intestinal permeability [18]. Apart from these solvents, the solubility of BCT in other solvents or its stability in solution have not been studied systematically.
Moreover, some analytical methods have been developed to quantify BCT, such as high-performance liquid chromatography (HPLC) and liquid chromatography–mass spectrometry (LCMS/MS). However, despite their advantages such as repeatability, high sensitivity, and reliability, HPLC methods imply a high cost, complex data processing, and greater time consumption. In contrast, UV/Vis absorption spectroscopy or fluorescence spectroscopy which has advantages over other methods, such as its ease and simplicity, fair sensitivity, relatively low cost, and low time consumption [19,20,21]. Although few analytical methods using these techniques have been developed for BCT [22], the absorption range of absorption wavelengths of BCT in the UV region implies high interference from other compounds, especially biological components, for which it limits the quantification of BCT from biological samples.
Furthermore, fluorescence spectroscopy on the other hand is a suitable and simple, inexpensive, rapid, and reproducible technique used for evaluating fluorescent compounds [23]. Compared to absorption spectroscopy, fluorescence spectroscopy is much more sensitive, permitting much lower limits of detection (LOD) and limits of quantification (LOQ). Because of the low solubility of BCT, the assessment of its in vitro availability in different tissues upon topical administration might require highly sensitive techniques such as this one.
Transcutol, also called diethylene glycol monoethyl ether, is a polymer of three units of ethylene glycol, and it is known to be a biocompatible solvent and permeation enhancer [24]. It is a clear and colorless liquid, which is water-soluble with a melting point of −76 °C [25]. It is widely used in pharmaceutical products, cosmetics, and food because of its low toxicity and high capacity as a solubilizer. Transcutol has been used in oral and sublingual solutions, as well as in injectable products. It has been included in creams, emulsions, gels, ointments, and solutions for topical delivery, covering a broad range of drugs and applications, including analgesic, anti-inflammatory, antifungal, hormones, and veterinary products [26]. Despite these advantages, Transcutol has never been used as a solvent for BCT in any studies. We should take into account that baricitinib presents severe side-effects, and the topical route is an alternative to the oral one, especially when local effects are intended. For instance, patients with atopic dermatitis who do not require systemic immunosuppressant therapy might benefit from topical formulations because this route may avoid systemic side-effects.
The aims of this study were firstly (i) to test the solubility of BCT in different solvents (aqueous solutions, oils, surfactants, and permeation enhancers such as Transcutol). It was to be a preliminary and pre-formulation study before leading to further developing formulations for topical delivery; (ii) to evaluate the uptake of the drug in different tissues (buccal, sublingual, nasal, vaginal, corneal, scleral mucosae, and skin) and the drug recovery; and (iii) to validate the absorption and fluorescence spectroscopy methods. As part of the validation, the stability of BCT solutions in Transcutol was studied at different temperatures.
Download the full article as PDF here Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies
or read it here
Materials
The BCT bulk powder ingredient was supplied by Henrikang Biotech Co., Ltd. (Xi’an, China). Transcutol, Labrafac, Isostearyl Isostearate, Labrasol, Lauroglycol 90, Labrafil M 1944 CS, Plurol oleique, and Capryol 90 were acquired from Gattefossé (Saint-Priest, France). Dimethyl sulfoxide (DMSO), Tween-80, and oleic acid were provided by Panreac Química SA (Barcelona, Spain); limonene, α-pinene, nonane, 1-decanol 99%, octanoic acid, lauryl sulfate, sebacic acid, castor oil, and phosphate-buffered solution pH 7.4 were purchased from Sigma Aldrich (St. Louis, MO, USA); Surfadone LP 100, N-ethyl pyrrolidone (NEP), and N-methyl pyrrolidone were obtained from ISP (West Yorkshire, UK). Perhydro Squalene was acquired from Fagron Iberica (Terrassa, Spain), liquid paraffin was supplied by Roig Farma, (Terrassa, Spain), and distilled water and purified water were obtained using a Station 9000 purification unit.
Mohammadi-Meyabadi, R.; Beirampour, N.; Garrós, N.; Alvarado, H.L.; Limón, D.; Silva-Abreu, M.; Calpena, A.C.; Mallandrich, M. Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies. Pharmaceutics 2022, 14, 2714. https://doi.org/10.3390/pharmaceutics14122714

